Alternative Complex III - ACIII
Despite the large diversity and flexibility observed in the prokaryotic electron transfer chains, the cytochrome bc1 complex family was thought to be the only able to perform quinol: electron acceptor oxidoreductase activity. However, in our group we have identified for the first time an alternative complex III, ACIII, which is structurally unrelated to the former.
ACIII from R. marinus is a seven subunit complex; three peripheral proteins comprising two c-type cytochromes, a multiheme and a monoheme one; a large subunit containing FeS centers; and four transmembrane proteins (Figure 1). The interaction of ACIII with quinol was established and at least one quinol binding site is present. We have also observed that ACIII is able to reduce a high potential iron-sulfur protein (HiPIP), a cytochrome c and can establish a functional association with the caa3 oxygen reductase.
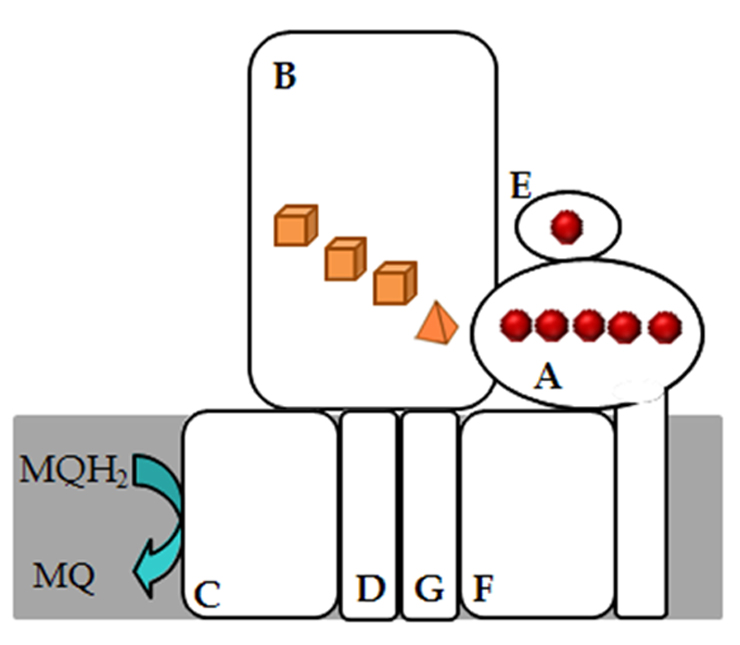
Figure 1 - Schematic representation of alternative complex III from R. marinus. The red spheres represents the c-type hemes and pyramides and cubes represents [3Fe-4S]1+/0 and [4Fe-4S]2+/1+, respectively.
Most important, the presence of ACIII is not exclusive of R. marinus; in fact, genes coding for this complex are widespread in the Bacteria domain being mostly present in genomes where the genes coding for the subunits of a typical complex III are absent and for which the presence of a complex with that activity is predicted. We have preformed a thorough analysis of genes and gene clusters coding for ACIII subunits, as well as an exhaustive analysis of the primary structures of the different subunits and their comparison with related proteins. We observed a relation of the ACIII with members of the complex iron-sulfur molybdoenzyme family and we concluded that ACIII is a new complex composed by a novel combination of modules already identified in other respiratory complexes. Despite the presence of the gene cluster coding for ACIII in many genomes, so far the enzymes from R. marinus and C. aurantiacus are the only complexes that were isolated and characterized.



