[Frontier Leaders] Relevance of Carbene Bonding Motifs in Enzyme Reactivity
Martin Albrecht, University College, Dublin, Ireland
| When |
04 Nov, 2013
from
12:00 pm to 01:00 pm |
|---|---|
| Where | Auditorium |
| Add event to your calendar |
|
Frontier Leaders Seminar
Title: Relevance of Carbene Bonding Motifs in Enzyme Reactivity
Speaker: Martin Albrecht
Affiliation: School of Chemistry & Chemical Biology, University College Dublin, Dublin, Ireland
Abstract:
Histidine is ubiquituous in metalloenzymes and generally considered to bind via one of its two side chain nitrogen atoms. Since the imidazole residue of histidine constitutes a direct precursor to N-heterocyclic carbenes, we were interested to investigate the impact of carbene bonding on the (catalytic) activity of metals in a biorelevant scaffold.
Towards this end, we will present a bottom-up strategy for the synthesis of organometallic petides and peptide mimetics. One approach entails the functionalization of histidine to investigate oligopeptides as active site mimics (see Scheme).[1]
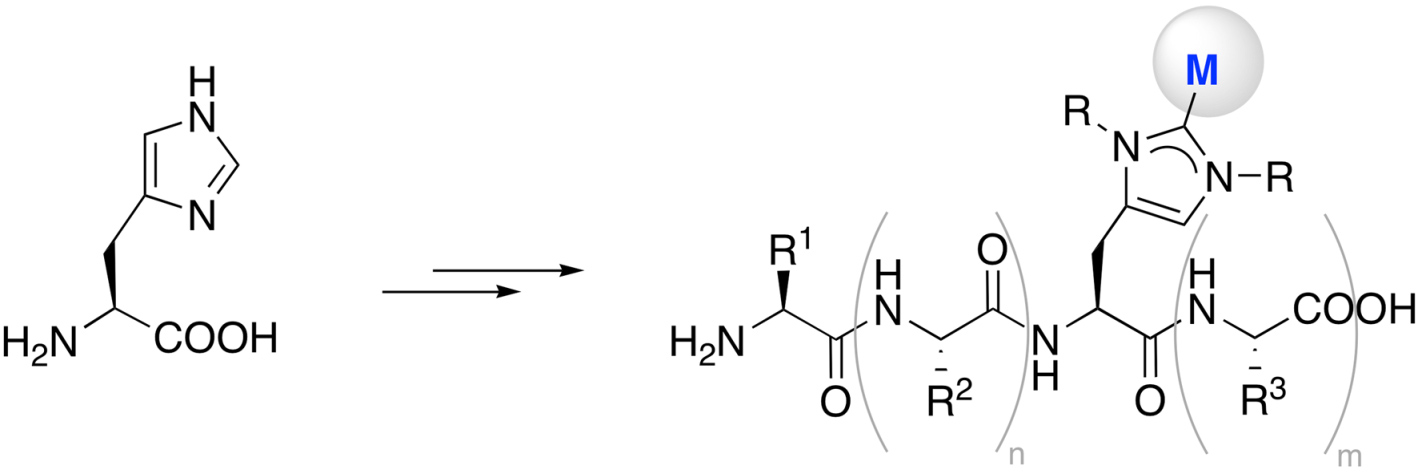
Furthermore, we will discuss results from site-directed mutagenesis)[2] as well as approaches towards functional analogues of (metallo)enzymes, including methyl transferase and the oxygen evolving center in photosystem II).[3] The latter approach has been particularly stimulated by parallel activities in our laboratories to develop strongly donating (abnormal) carbenes).[4]
References:
[1] A. Monney et al. Dalton Trans. 2011, 40, 2716; Chem. Commun. 2012, 48, 10960. Dalton Trans. 2013, 42, 5655.
[2] G. Gucciardo et al., submitted.
[3] For examples: R. Lalrempuia et al. Angew. Chem. Int. Ed. 2010, 49, 9765. L. Bernet et al, Chem. Commun. 2011, 47, 8058. R. Lalrempuia et al. Angew. Chem. Int. Ed. 2011, 50, 9969. J. Olguin et al. submitted.
[4] O. Schuster et al. Chem. Rev. 2009, 109, 3445; P. Mathew et al. J. Am. Chem. Soc. 2008, 130, 13534.







