What we study
What do we study
1- Oxygen detoxification
Oxygen by itself is a toxic species, due to its high reduction potential, being able to oxidize a plethora of cellular constituents. Therefore, organisms that continuously are exposed to oxygen in their environment, or even produce it endogenously (oxygenic organisms), as well as anaerobes that either in the external environment or inside the host are exposed transiently to deadly concentrations of oxygen, are endowed with enzymes to directly reduce it to water. Apart from the well-known respiratory and membrane bound oxygen reductases, a family of cytoplasmatic enzymes exists that is able to convert O2 into H2O. These enzymes - the flavodiiron proteins (FDPs)- contribute to combat oxidative stress by, simultaneously, eliminating oxygen and, indirectly, decreasing the formation of reactive oxygen species. FDPs have the capability of reducing O2 to water and/or NO to the innocuous N2O. Flavodiiron proteins are widespread in the three life Kingdoms and are metalloenzymes having as the minimal core unit a diiron-containing and an FMN containing domains, although much more complex FDPs are encountered in the microbial world, having a diversity of extra iron- and flavin- containing modules.
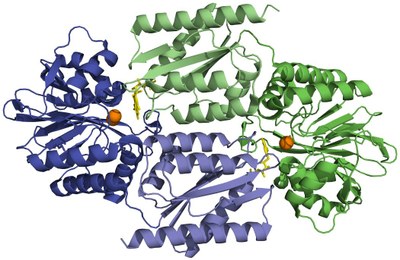 |
| Cartoon representation of the homodimeric D. gigas FDP (PDB code 1E5D) crystallographic structure. One monomer is represented in dark and light green, the other in dark and light blue. Darker colours assign the N-terminal domain with a ß-lactamase-like fold, lighter colors assign the C-terminal domain with a flavodoxin fold. The diiron center (orange spheres) of one monomer is placed in the vicinity of the FMN group (yellow sticks) of the other monomer, owing to the head-to-tail arrangement of the dimer. |
2- NO detoxification
Another aspect of oxidative and nitrosative stress concerns the mammalian immunological defence mechanisms against pathogen invasion and infection. Activated macrophages are known to produce nitric oxide, a highly reactive and cytotoxic radical molecule which inhibits and destroys several cellular functions. NO can also be produced abiotically, through the reduction of nitrite to NO in acidic media (e.g., in the human stomach), or biotically, as an intermediate of microbial denitrification pathways (e.g., by human gut microbiota components). Pathogens, such as E. coli and Clostridiales (e.g., Clostridium difficile) have thus evolved counteracting mechanisms to subvert the host's immune system, namely through the scavenging of nitric oxide. The above mentioned flavodiiron proteins have been demonstrated to also act, in some cases, as nitric oxide reductases, therefore eliminating it. In our Laboratory, we study the relevance of flavodiiron proteins from several organisms, namely human pathogens, for NO detoxification.
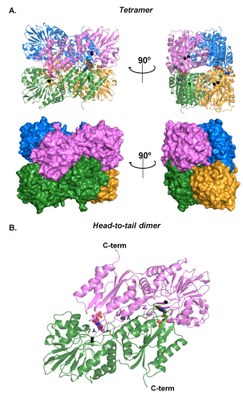 |
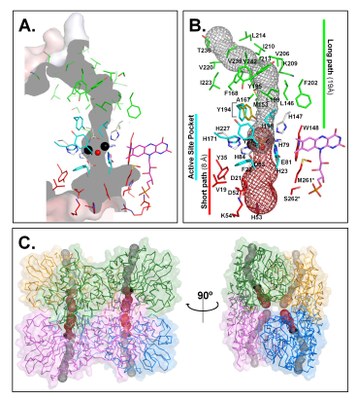 |
| E. coli FDP (flavorubredoxin) NO reductase structure (PDB 4D02) | FlRd substrate (NO, O2) channels |
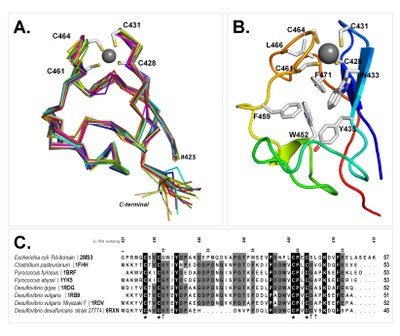 |
FlRd rubredoxin domain NMR structure of the zinc form |
3-Superoxide detoxification
Superoxide anion is a toxic radical generated in living systems whenever they are exposed to molecular oxygen. Even though the formation of the superoxide anion from molecular oxygen is thermodynamically unfavourable, inside the highly reductive cell medium, superoxide is produced due to the presence of catalysts such as transition metals and flavins. Superoxide plays a key role in a large number of diseases including cardiovascular and neurodegenerative diseases, aging and cancer. To deal with oxidative stress, both aerobes and anaerobes, that face continuous or transient exposure to oxygen, have developed specialized detoxifying systems.
Some organisms explore superoxide's killing potential in their own benefit, using it as a weapon against invading pathogens. This is especially important in the mammalian immune system, where the macrophages, activated by the presence of the pathogen, produce superoxide upon phagocytosis.
Recently a new type of activity against superoxide was found in anaerobic and microaerophilic prokaryotes (including numerous pathogens), as well as in a few Eukarya: the superoxide reductases (SOR). In our laboratory, these iron enzymes from several microbial sources are studied as part of the defence mechanism against superoxide.
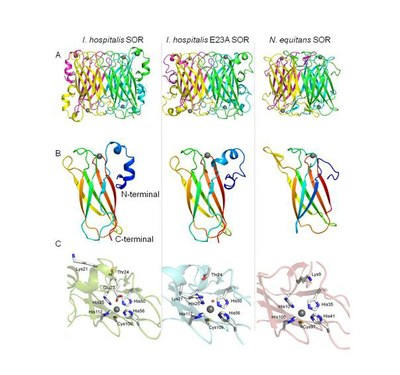
Three-dimensional crystal structures of the SORs from Ignicoccus hospitalis, wt and E23A mutant proteins, and of the Nanoarchaeum eequitans SOR obtained by X-ray crystallography. A) Tetrameric quaternary structure for the three SORs, composed by a pair of two antiparallel subunits; the four subunits are colored differently; B) SORs monomer structure, each monomer is colored from blue (N-terminal) to red (C-terminal); C) SORs oxidized active center. Iron ion is represented as gray sphere, a water molecule is in a red sphere, protein secondary structure is represented as in cartoon and the amino acid side-chains are represented as sticks.
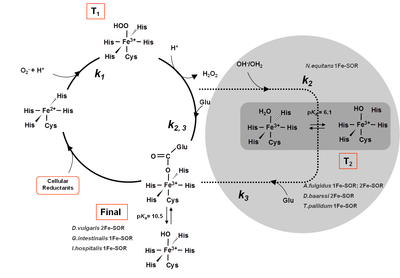
Mechanism of the catalytic reduction of superoxide proposed for different SORs, evidencing the detected intermediates for each case. The N.equitans SOR appears inside the grey circle, due to the absence of the glutamate residue.
4- Rubrerythrins – H2O2 detoxification
Rubrerythrins were discovered in the eighties, but for a long time their function remained unknown. They were so named because they contain two types of iron sites: a diiron center, reminiscent of that found in hemerythrins (oxygen carrying proteins from worms), and a rubredoxin-like FeCys4 center. The determination of its 3D crystallographic structure revealed that the diiron site is present in a four helix bundle domain, which is followed by a rubredoxin-like domain at the C- or N-terminus, where the second iron center is located. The ligands of the iron ions of the diiron center are histidines and carboxylates (aspartates and glutamates), i.e., the rubrerythrins belong to the large superfamily of four helix bundle proteins containing a diiron centre bound to His and Asp/Glu residues. The proteins of this superfamily have important functions, such as the ribonucleotide reductases, the methane monooxygenase, and the ferritins. All these enzymes have in common their ability to react with molecular oxygen. The function and mechanism of rubrerythrins was a matter of dispute, with several functions proposed. So far the most "consensual" enzymic function for rubrerythrins is that of hydrogen peroxide reductases. In vivo experiments seem to corroborate this hypothesis, since they show that the transcription of the rubrerythrins genes changes upon oxidative stress conditions. However, in a situation reminiscent to that of the flavodiiron reductases (which may be NO reductases, O2 reductases, or both), there are also reports of the importance of rubrerythrins in protection against nitrosative stress; so far no direct evidence of any type of reactivity against nitrogen reactive species has been reported, but it should be stressed that diiron enzymes in general are known to bind NO molecules. In our Laboratory we study rubrerythrins from various sources, namely from the pathogenic Clostridia species.
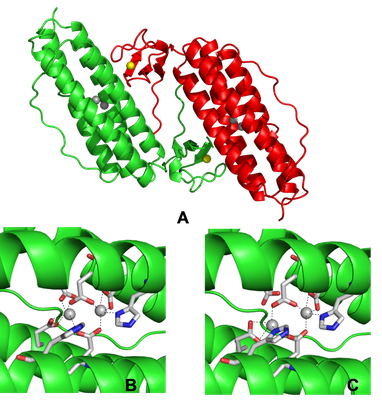
3D crystal structure of Rubrerythrin from Desulfovibrio vulgaris (A) homodimer pdb 1RYT, (B) All-ferric diiron center pdb 1LKM and (C) All-ferrous diiron center pdb 1LKO. Iron atoms from the diiron center are represented in gray spheres and the iron atoms from Rd domain are represented in yellow spheres.
All the enzymes under study have a modular structure, which may be very complex, being constituted by a small number of building blocks, namely containing iron and flavin cofactors.
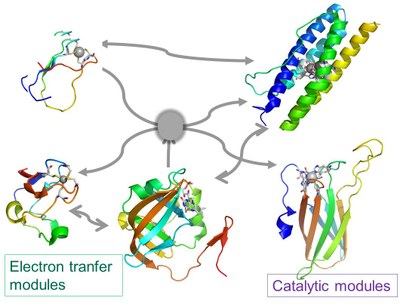
Modular nature of some of the metalloenzymes under study involved in oxidative and nitrosative stress defences
5- Other metalloenzymes
In collaboration with other groups we also study other metalloproteins, such as multiheme cytochromes, endonucleases, proteins involved in FeS biogenesis, globins, among others.



