Oxygen toxicity
Dioxygen Toxicity
Reactivity of Molecular Oxygen
Molecular oxygen is a powerful oxidizing agent, and the energy that fuels most nonphotosynthetic biology is obtained by reducing it to two water molecules in enzyme-catalysed reactions (aerobic respiratory chains). However, this molecule is relatively inert because its most stable form is a spin triplet state. That means that it possesses two unpaired electrons, and to receive two electrons simultaneously it must occur a forbidden spin transition. The consequence is that simultaneous two-electrons reduction by the common biological singlet molecules is not favourable in the absence of catalysts. Therefore dioxygen is usually constrained to one electron transfer at a time. This leads to the formation of superoxide radicals, hydrogen peroxide, and hydroxyl radicals. These are commonly called reactive oxygen species (ROS), and participate in a wide variety of oxidative damage reactions.
How are superoxide and hydrogen peroxide produced in the cell?
The one-electron reduction of dioxygen to superoxide is thermodinamically and kinetically unfavourable. A way to overcome the spin restriction lies in the interaction of the dioxygen molecule with another paramagnetic molecule. Transition metals, such as Fe or Cu, frequently have unpaired electrons and are therefore excellent catalysts for dioxygen reduction. Superoxide can also be produced by univalent electron reduction of oxygen by organic compounds which have the ability to give an electron at a time. Flavin cofactors present in many electron transfer proteins meet these criteria. Important sources of these enzymes are the respiratory chains. During the normal turnover of respiratory dehydrogenases, which use flavin cofactors to accept hydride anions from organic substrates, electrons are transfered one at a time onto secondary redox moieties, either iron sulfur clusters or bound quinones. Therefore, a reaction of dioxygen with a reduced flavin may lead to the formation of superoxide. Superoxide is ultimately transformed to hydrogen peroxide and dioxygen by self-dismutation, or more efficiently, catalized by superoxide dismutases (SODs) at a diffusion controlled rate, or may be reduced by superoxide reductases, to form hydrogen peroxide.
What are the biological effects of superoxide?
Although the mechanisms of the biological toxicity of superoxide are not yet fully understood, the most striking proof of its deleterious effects came from the evidence that superoxide dismutase (SOD) deficient E. coli mutants have impaired growth when exposed to high concentrations of oxygen. Nevertheless, some targets are already identified. One of them is the solvent exposed [4Fe-4S] cluster of dehydratases, e.g. aconitase (a key enzyme of the tricarboxylic acid cycle). Oxidation of this centre by superoxide, results in loss of the catalytic iron atoms, enzyme inactivation, and interruption of the metabolic pathway. Another pathway inactivated by superoxide is the synthesis of aromatic amino acids. Superoxide can also react with trioses to form methyl glyoxal, a toxic compound which attacks amino groups of proteins and nucleic acids.
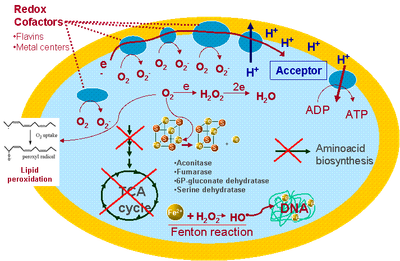 |
| Figure 1- Production of superoxide within a cell |
What are the biological effects of hydrogen peroxide?
Cellular targets of hydrogen peroxide have not been yet clearly identified, but it can oxidize enzyme thiols and thus is likely to inactivate enzymes that rely upon active-site cysteinyl residues for catalytic function; it also oxidizes many metalloproteins. Hydrogen peroxide can react with reduced iron through the Fenton reaction to produce the highly toxic hydroxyl radical:
| H2O2 + Fe2+ → Fe3+ +OH- + OH. |
The hydroxyl radical is the only species that can directly damage most biomolecules. This radical oxidises most organic molecules at diffusion-limited rates. These reactions are so fast that the oxidative damage promoted by OH· occurs in a site-specific manner, which is to say that once formed, it will react immediately with a molecule in the vicinity of the site of formation. DNA is very good in chelating iron, therefore it's a favoured target for Fenton-mediated damage.
Bibliography
Cadenas, E. (1989). "Biochemistry of oxygen toxicity." Annu Rev Biochem 58 : 79-110.
Gardner, P. R. and I. Fridovich (1991). "Superoxide sensitivity of the Escherichia coli aconitase." J Biol Chem 266 (29): 19328-33.
Imlay, J. A. (2003). "Pathways of oxidative damage." Annu Rev Microbiol 57 : 395-418.
Valentine, J. S., D. L. Wertz, et al. (1998). "The dark side of dioxygen biochemistry." Curr Opin Chem Biol 2 (2): 253-62.
Sheng Y, Abreu IA, Cabelli DE, Maroney MJ, Miller AF, Teixeira M, Valentine JS., 2014, Superoxide dismutases and superoxide reductases., 2014, Chem Rev. 114:3854-3918.



