Stem cells bioprocessing and cell therapy
Stem cells (SCs), with their ability for extensive proliferation and multi-lineage differentiation, can serve as a renewable source of cellular material in regenerative medicine and toxicology. A pre-requisite for the transition of SCs, or their progeny, to these fields is the establishment of efficient cell culture protocols for large-scale expansion, differentiation, storage, and distribution. This efficiency will be measured in terms of robustness, reproducibility, scalability, and automation, as well as compatibility with ATMP (Advanced Therapy Medicinal Products) regulations from EMA, and compliant with current Good Manufacturing Practices (cGMP).
While the potential for producing novel cell-based products is large, there are still major challenges in producing SC-derivatives, such as:
- the scaling up of reproducible pure cell populations of undifferentiated cells without compromising their self-renewal ability and differentiation potential.
- the directed differentiation to specific cell types with improved differentiation efficiency, high purity, and cell functionality.
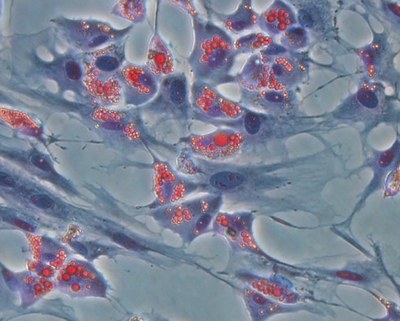
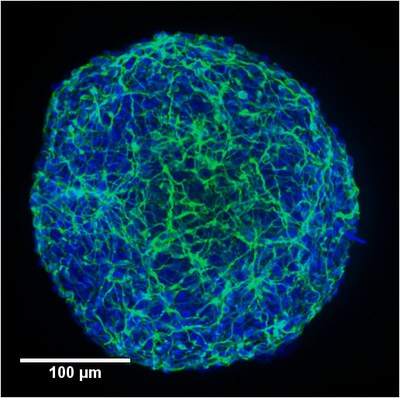
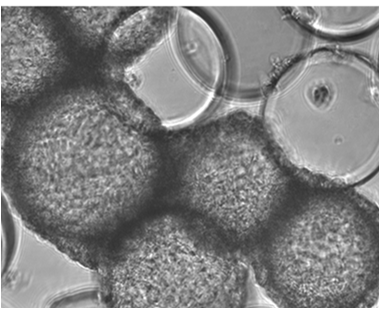
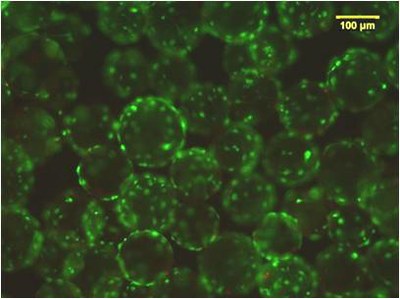
At the Animal Cell Technology (ACT) Unit, we are applying our expertise in bioreactor technology to the development of effective and scalable culture systems that can integrate stem cell in vitro expansion, their target differentiation, and cryopreservation. We have been using stirred-tank bioreactors, that allow process scale-up and automation, as well as efficient monitoring and control of the culture environment. For on-line and non-invasive phenotypic monitoring and selection, our group is developing new reporter human embryonic SC lines.
Furthermore, by integrating new approaches for cryopreservation, it will be possible to distribute high quantities of ready-to use cells with improved viability and functionality. We are currently working with embryonic and adult stem cells, of human and murine origin.