Development of nanoparticles for drug delivery
Technological improvements have made possible the production of a large number of new bioactive agents (BAs) (e.g. antioxidants, therapeutic proteins) important to the pharmaceutical and medical applications. Transdermal drug delivery system is defined as the topically administered medications, which when applied to the skin, deliver the drug through the skin at a predetermined and controlled rate. Transdermal delivery of BAs avoids the disadvantages associated with the invasive parenteral route of administration and other alternative routes such as the pulmonary and nasal routes. The evaluation of percutaneous absorption of BAs is an essential for optimizing the delivery of BAs of pharmaceutical and cosmetic importance, for toxicology and risk assessment of these materials and for estimating occupational exposure and similar hazards. Delivery of nanocarriers provides a mean to improve and control stability, activity, solubility and bioavailability as well as controlled release and targeting of BAs. An established strategy to drug delivery consists of attaching the drugs to suitable nanocarrier system and as a result, there is an increased therapeutic index by controlling the rate and site of drug release. The type of nanocarriers, and related physicochemical and morphological properties are closely related with the type of delivery system.
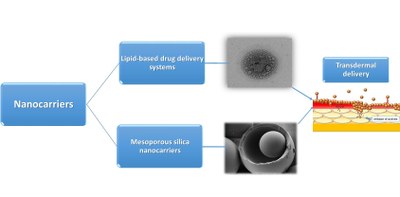
The skin permeability studies from physicochemical characteristics of BAs (hydrophilic and hydrophobic) in correlation with drug delivery system (type, morphology, size and surface charge of nanoparticles) contribute for better understanding of the mechanism responsible for partition and binding properties through SC, and the factors which govern partition.



