RUVBL
RUVBL Highlight
The Crystal Structure of the Human AAA+ Protein RuvBL1
RuvBL1 is an evolutionarily highly conserved eukaryotic protein belonging to the AAA+-family of ATPases (ATPase associated with diverse cellular activities). It plays important roles in essential signalling pathways such as the c-Myc and Wnt pathways, in chromatin remodelling, in transcriptional and developmental regulation, and in DNA repair and apoptosis.
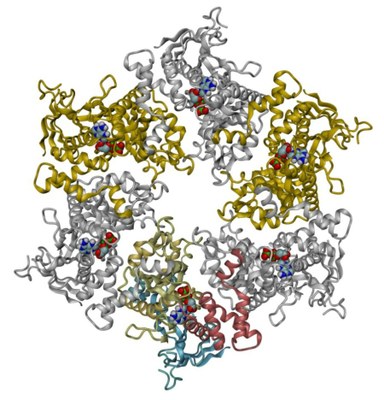 | 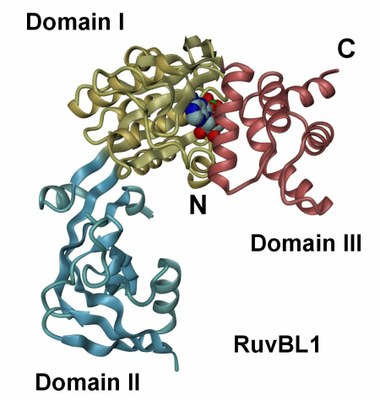 |
The crystal structure of RuvBL1 consists of hexamers, formed of ADP-bound monomers. The hexamers exhibit a central channel ca. 18 Å in diameter | The RuvBL1 monomer contains three domains, of which the first and the third are involved in ATP binding and hydrolysis. |
| 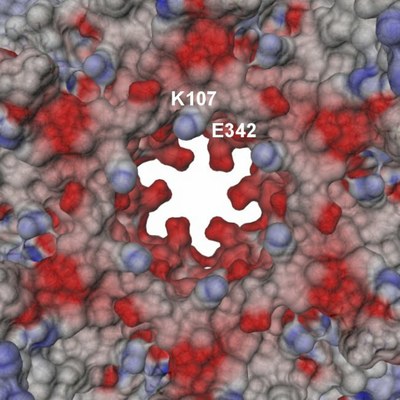 |
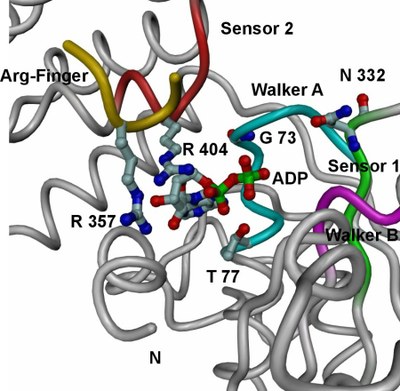
| Tube view of RuvBL1 in the region of the nucleotide-binding pocket. | View of the electrostatic potential of the RuvBL1 hexamer mapped at the molecular surface in the region of its central channel. |
The structure of the RuvBL1/ADP complex, combined with our biochemical results, suggest that while RuvBL1 has all the structural characteristics of a molecular motor, even of an ATP-driven helicase, one or more as yet undetermined co-factors are needed for its enzymatic activity. |
References: P. M. Matias, S. Gorynia, P. Donner, M. A. Carrondo “Crystal Structure of the Human AAA+ Protein RuvBL1” (2006) J. Biol. Chem., JBC published October 23, 2006 as doi:10.1074/jbc.M605625200 [Accepted Manuscript] |