News
NOVA Science & Innovation Day 2025
12 December 2025
We have presented our project, Next Generation Natural Sweeteners.
This project aims to engineer oxidative enzymes for the biotechnological production of rare sugars (RS), increasingly valued as natural sweeteners for food and nutraceutical applications. RS offer substantial advantages over plant-derived Stevia, whose production is limited by climate and agricultural constraints, because they are nearly zero-calorie, have minimal glycemic impact, and provide additional health benefits.

Biocatalysis Division of the European Federation of Biotechnology (EFB)
8 December 2025
Lígia Martins has been appointed Co-Chair of the Biocatalysis Division of the EFB, together with Tom Clarke (University of East Anglia, UK), contributing to scientific excellence and collaboration in biocatalysis across Europe.
Sociedade Portuguesa de Biotecnologia (SPBT)
Lígia Martins is now part of the new SPBT Board, with Lucília Domingues (University of Minho) as President and Jorge Pereira (University of Coimbra) as Vice-President, helping shape the future of biotechnology in Portugal.
Ponta Delgada, S. Miguel, Açores, 5 December 2025

Congress of Microbiology and Biotechnology MICROBIOTECH25
Ponta Delgada, S. Miguel, Açores, 4-6 December 2025
Our group was actively represented at the meeting, providing an excellent opportunity to share ideas, discuss ongoing work, and strengthen scientific interactions. Lígia O. Martins delivered a Keynote Lecture, while André Taborda and Carolina Rodrigues presented oral communications, and Vânia Brissos and Paulo Durão contributed poster presentations, showcasing ongoing research activities and fostering valuable discussions on current and future research directions.

We are proud to have contributed our data from directed evolution engineering of enzymes to a novel database of experimental thermostability data for single-point mutants, FireProtDB, designed to facilitate both types of expected use: the interactive exploration of individual entries at the level of a protein or mutation and the construction of highly customized, machine-learning-friendly datasets through advanced searching and filtering.
November, 2025

Read the paper here: Musil, M, Borko, S, Plana-Iglesias, J, Lacko, D, Rosinska, M., Kabourek, P, Martins, LO., Tataruch, M, Damborsky, J, Mazurenko, S, Bednar, D. 2025. FireProtDB 2.0: large-scale manually curated database of the protein stability data. Nucleic Acids Res. gkaf1211
15th ITQB NOVA PhD Student Meeting
Carolina and Tiago presented and discussed their research with fellow students, postdocs, and senior scientists. This student-organized event fosters scientific exchange and collaboration across the ITQB NOVA community, providing a dynamic platform for young researchers to share ideas and receive valuable feedback.
Oeiras, 22-24 October 2025

Don't miss our collaborative work "In operando assessment of 2O2 production by a multicopper oxidase under direct electron transfer with a gold electrode" by Panpan Wang, Vânia Brissos, Lígia O. Martins, Wolfgang Schuhmann and Felipe Conzuelo.
22nd October 2025

In this communication, the localized, real-time characterization of a multicopper oxidase, McoA variant from Aquifex aeolicus, adsorbed onto a gold electrode was performed using a dedicated microbiosensor by the Conzuelo group at ITQB-NOVA. This provided detailed information on the O2 reduction, which is shown to proceed without detectable H2O2 accumulation. Instead, H2O2 originates from direct O2 conversion at the gold surface.
Our project, NEXT-GENERATION NATURAL SWEETENEERS, a collaboration with André Taborda and Rita Ventura, was one of the winning projects of the 4th edition of the IOV Proof of Concept (PoC) Fund. This program supports promising early-stage research projects (with a low Technology Readiness Level, or those in their journey toward real-world application). The institutional partners in the IOV PoC are ITQB NOVA, NIMSB - NOVA Institute for Medical Systems Biology, INIAV, IP - Instituto Nacional de Investigação Agrária e Veterinária, and the Câmara Municipal de Oeiras.
This award was presented during BIOMEET 2025, the ideal platform celebration, organized by the P-BIO - Associação Portuguesa de Bioindústria.
Oeiras, 29 Sep 2025

https://www.nowcanal.pt/programas/global-5-0/detalhe/global-5-0-temporada-2-ep-45
Attendance at the 14th Int Conference on Protein Stabilization ProtSatb25
Timișoara, Romania, 21-25 Oct 2025
Lígia Martins delivered a Plenary Lecture on "Network Dynamics as Fingerprints of Thermostability in an In Silico-Engineered DyP-type Peroxidase" and Patricia T. Borges, presented a poster on the same topic.

Highlight on the ITQB-NOVA website:
Standing the heat: strategies shaping sustainable bioprocesses
Oeiras, 8 sep 2025

Highlight in ITQB NEWSLETTER:

In our latest paper Network Dynamics as Fingerprints of Thermostability in an In Silico-Engineered DyP-type Peroxidase, we propose that apart from the traditional protein thermostable-associated feature s obtained by static structural analysis, including increased compactness, rigidity, and an enriched network of hydrogen bonds and hydrophobic interactions, by integrating dynamic modeling with protein correlation network analysis a previously unrecognized fingerprint of stabilization was suggested: a highly connected structural networks characterized by denser and more persistent intra- and inter-monomer interactions, greater internal cohesion, and enhanced cooperative dynamics is present in thermostable proteins as compred with their mesophilic counterparts. DyP enzyme tetramers exhibit long-range communication pathways and redundant routes, supporting coordinated motions that can hinder local unfolding and tetramer dissociation.
s obtained by static structural analysis, including increased compactness, rigidity, and an enriched network of hydrogen bonds and hydrophobic interactions, by integrating dynamic modeling with protein correlation network analysis a previously unrecognized fingerprint of stabilization was suggested: a highly connected structural networks characterized by denser and more persistent intra- and inter-monomer interactions, greater internal cohesion, and enhanced cooperative dynamics is present in thermostable proteins as compred with their mesophilic counterparts. DyP enzyme tetramers exhibit long-range communication pathways and redundant routes, supporting coordinated motions that can hinder local unfolding and tetramer dissociation.
Tiago Lopes & Pedro Almeida attended the Gordon Research Conference on Carbohydrate-Active Enzymes for Glycan Conversions at Proctor Academy, New Hampshire, USA
July 20-25, USA, 2025
These conferences are focused on advancing the frontiers of science by presenting cutting-edge, unpublished research, prioritizing time for discussion after each talk, and fostering informal interactions among scientists at all career stages.


Elena Potocka presented her work in BIOTRANS 2025, 17th International Symposium on Biocatalysis and Biotransformations!
Basel, June 29-July 3, 2025
Elena discussed progress on the sustainable chemo-enzymatic strateg y using glycoside-3-oxidase to produce rare sugars with health-promoting potential, enhancing both the diversity and eco-friendliness of sweetener synthesis. This work is a collaboration with the Lab of Rita Ventura. Congrats, Elena!
y using glycoside-3-oxidase to produce rare sugars with health-promoting potential, enhancing both the diversity and eco-friendliness of sweetener synthesis. This work is a collaboration with the Lab of Rita Ventura. Congrats, Elena! ![]()
We have participated in the LS4FUTURE 2nd General Meeting. Over 200 participants came together to explore the challenges of collaborative research, identify synergies, and shape strategic priorities for the coming years. The program of the featured scientific presentations, poster sessions, and thematic workshops focused on the LS4FUTURE Research Lines, with a special emphasis on the role of artificial intelligence in advancing science.
Oeiras July 1, 2025

Margarida Monteiro presented her Bachelor's Project on the successfull Engineering of Galactose Oxidase!
Caparica, June 18, 2025
Margarida, a BSc student in Cellular & Molecular Biology (FCT-NOVA), joined the M&ET Lab for her bachelor project, taking on the challenge of engineering Galactose Oxidase for gluco se oxidation under the supervision of Tiago Lopes. She recently presented her work to the university jury as the final step of the project, highlighting promising progress and delivering it with great enthusiasm. Well done, Margarida!
se oxidation under the supervision of Tiago Lopes. She recently presented her work to the university jury as the final step of the project, highlighting promising progress and delivering it with great enthusiasm. Well done, Margarida! ![]()
Inês Corrêa Presented her Innovative Mycotoxin Research at BioPic Forum 2025!
Lisboa, June 16, 2025
Inês stayed for a short training period at our lab as part of a CU "Integrated 1st Cycle Project in Biological Engineering" at Instituto Superior Técnico, where she performed work focused on "Laccase-Mediated Degradation of Multiple and Major Mycotoxins”, under the supervision of Vânia Brissos, which was showcased at the BioPic Forum 2025 at the Técnico Innovation Center. Her research highlights promising enzymatic approaches to tackle food contaminants. Well done, Inês! ![]()

Pedro Almeida participated in the 2025 MIT Portugal Innovation Workshop, held at the Massachusetts Institute of Technology.
Part of the MIT Portugal Program, this hands-on workshop focuses on venture creation and the potential commercialization of high-impact research and technologies.
June 9-13, MIT, Boston, USA
Meeting of the COST Action COZYME Pan-European Network on Computational Redesign of Enzymes
Athens, Greece, 24-25 April 2025, at the National Technical University of Athens (NTUA), Patision Campus, organized by Prof Evangelos Topakas, Dr Christina Ferousi and their team.
We had oral sessions of WG 1, WG 2, and WG 3, poster sessions, and a session where researchers who benefited from short-term scientific missions (STSMs) presented their work, as in the case of PhD student André Taborda, who spent a period at Loschmidt Laboratories based at the Masaryk University at Brno, CZ, led by Prof. Jiri Damborsky.

The Cover Feature of ChemSusChem shows laccases as biocatalysts with high potential in lignocellulose biorefineries. Protein engineering has enhanced their efficiency for valorizing lignin monomers into value-added biologically active molecules. This biocatalytic process is solvent-free, achieves faster reaction times, uses lower amounts of enzyme, and delivers excellent yields (up to 100 %) of eco-friendly natural dimeric compounds for medicinal chemistry and polymer synthesis. More information can be found in the Research Article by V. Brissos, M. Rénio, M. P. Robalo, M. R. Ventura, L. O. Martins and co-workers (DOI: 10.1002/cssc.202401386). Cover design: Joel Arruda (ITQB-NOVA Science Communication Office).
4 April 2025

Catarina Coelho, Tiago Lopes, Tomás Frazão and Lígia Martins attended the NOVEL ENZYMES conference to learn and exchange ideas!
The 8th International Conference on Novel Enzymes provided an overview of the latest results and research directions related to new enzymes, the latest developments and the future trends of the field.
Budapest, Hungary, March 25-28, 2025

Our new project, MSCA-Doctoral Network, started on 1 March 2025, and we have posted 10 doctoral positions: please read it here, spread the word and apply https://msca-comenze.eu/

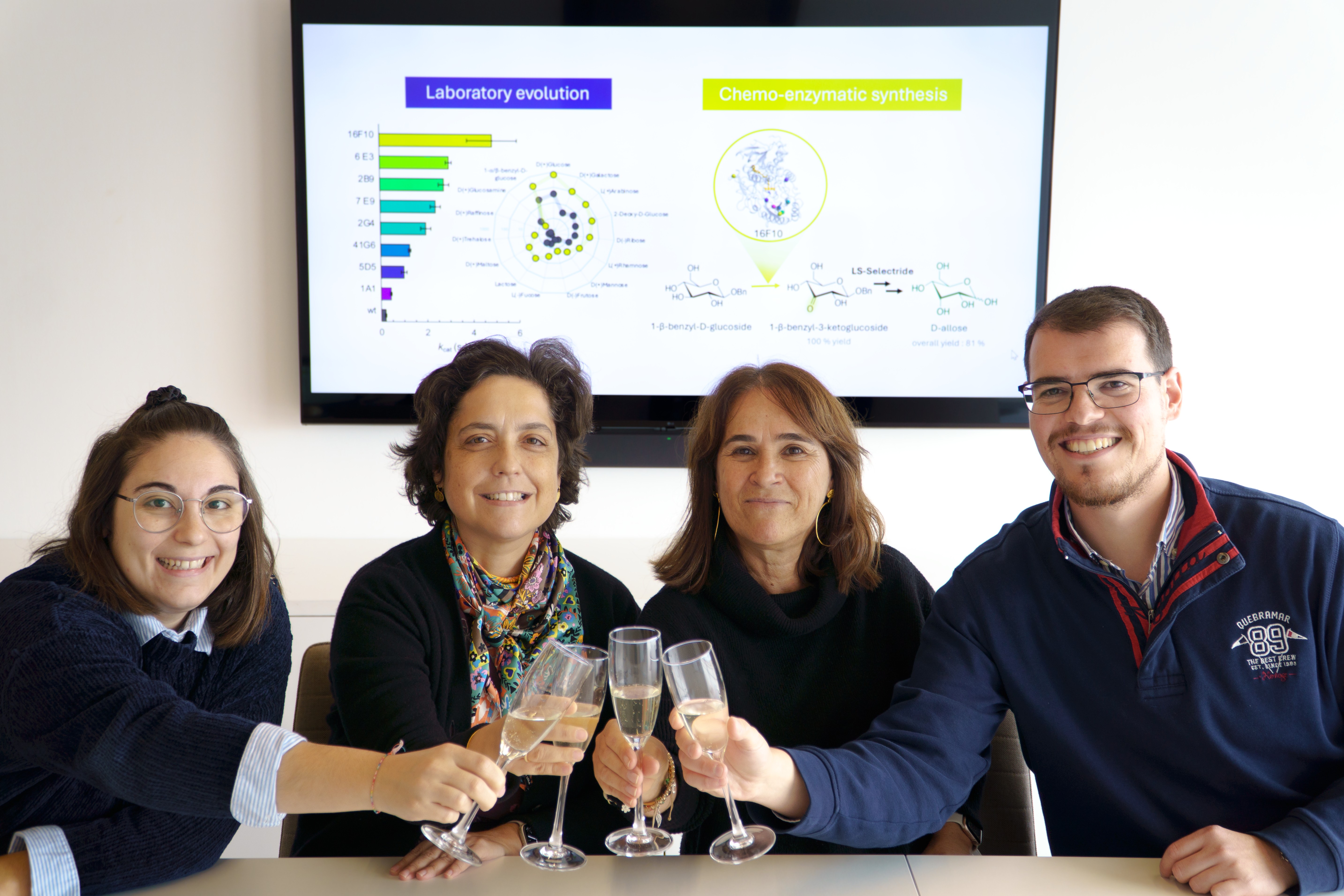
CELEBRATION!!![]() the workers behind the rare-sugar work;)
the workers behind the rare-sugar work;)
Highlight at ITQB-NOVA and in Media:
ITQB-NOVA: Sweet science: a new approach to synthesize rare health-packed sugars
HealthNews: Nova técnica para sintetizar açúcares raros promete revolucionar indústria alimentar
RTP notícias: Investigadores portugueses partem em busca de açúcar saudável
Meteored: É doce e faz bem à saúde: assim é o açúcar que esta equipa portuguesa inventou

BACK-COVER of our paper in Green Chemistry, 2025, 27, 1044 - 1053. The artwork was designed with Joel Arruda, Communication Office, ITQB NOVA.
This research showcases research from Lígia Martins and Rita Ventura Labs at ITQB NOVA. It highlights the transformative potential of enzyme engineering through directed evolution for biotechnological innovation. Using an engineered enzyme, the team developed a groundbreaking chemo-enzymatic method to synthesize allose - a rare sugar found in trace amounts in plants - starting from abundant and cost-effective compounds. Allose shows promise as a functional ingredient for applications in food, nutraceuticals, and pharmaceuticals. Read the paper HERE.
Kick-off meeting
McGEA: Metalloenzymes and Cells for Green Environmental Alternatives Staff Exchange. HORIZON-MSCA-SE-2023 (2024-2028)
Oeiras, 13 Dec 2024

Campus de Campolide, 3 Dec 2024

We attended Biocat4Value, a Biocatalysis Conference for Biological Transformation along Renewable Value Chains organized by the European Federation of Biotechnology (EFB) Biocatalysis Division.
The conference had a primary emphasis on networking, knowledge exchange, and collaborative partnerships; the Biocat4Value event drew participants from market leaders in biocatalysis, SMEs selected academic experts, and bioeconomy regions across Europe
18-20 November 2024 in Düsseldorf, Germany

Patrícia Borges, our structuralist Post-Doc, gave an exciting SCAN (Scientific Conferences at NOON) at ITQB: "Unravel structure and functional details of enzymes involved in lignin valorization"! ![]()
Oeiras, 20 November 2024

This week, we were at the ITQB-NOVA newsletter twice:)

2nd BioExcel Conference on Advances in Biomolecular Simulations
20-23 Oct 2024, Brno, Czech Rep
Our PhD student Tomás Frazão participated in this conference, which allowed him to learn the latest trends, updates and challenges in integrative modeling, free energy and drug design, workflows, automation and data integration. He said, "I really liked it; it was another dimension. We had 90 posters, but even so, I could see many and get an overall idea of what people were working on. We had a discussion, and people suggested two or three software options to solve a problem with my enzyme." he added, "Brno is small but very charming."!

Innovation Challenges: Researchers Impact Series
Ljubljana, Slovenia, 13th – 17th October 2024
Our PhD student Mario De Simone participated in the EUTOPIA Innovation Challenges in Ljubljana, a dynamic workshop that brought together top researchers from 10 European universities. The event provided a unique platform for collaboration with Slovenian companies and startups, where they tackled real-world challenges, sparking fresh ideas and forging innovative solutions. For Mario, it was an insightful experience that opened up opportunities for new collaborations and deepened his understanding of the impact and challenges of the circular bioeconomy, particularly in the olive oil industry.

14th ITQB NOVA PhD Student Meeting
André and Mario had the opportunity to present and discuss their work with ITQB NOVA scientists at a student-organized meeting.
Oeiras, 14-18 October 2024

We attended the Lignobiotech2024 conference in Toulouse, France, where we met old friends and made new ones and shared our work with the community that studies Lignocellulose Biomass Valorization! It was a fantastic meeting full of good science, great food;) and animation!
Toulouse, France, October 14- 17, 2024

Over the past three weeks, our PhD student André Taborda had the privilege of undertaking a Short-Term Scientific Mission funded by COZYME - COST action CA21162, where he focused on protein stability analysis, working closely with the technology team at Loschmidt Laboratories, Faculty of Science, Masaryk University, Czech Republic. Thanks to everyone for making him feel so welcome, especially Sérgio Marques, David Bednář, and Prof. Jiri Damborsky for their invaluable mentorship during his stay. Their guidance provided André with hands-on experience using some computational tools for protein stability analysis and sparked insightful discussions that broadened his understanding of the field.
Brno, Czech Republic, September 14- October 4, 2024

Lígia Martins, undertaking a Short-Term Scientific Mission funded by COZYME - COST action CA21162, was at the Faculty of Chemistry and Chemical Engineering, Babeș-Bolyai University, Cluj-Napoca, Romania, a member of the EUTOPIA alliance. This 4-day mission aimed to extend and strengthen our group's scientific collaboration in enzyme engineering with the Enzymology and Applied Biocatalysis Center members (particularly with Prof. Dr. Laszlo Csaba Bencze). This stay allowed the sharing of expertise/skills. It helped identify potential joint research interests and funding opportunities in enzyme engineering for future joint projects and students based on the complementary expertise of the two groups.
Cluj-Napoca, Romenia, September 24-29, 2024


Unlocking Lignin's Potential: Engineered Bacterial Laccases to Produce Biologically Active Molecules
Oeiras, 11 Sep 2024
Paper: https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cssc.202401386
ITQB Highlight: https://www.itqb.unl.pt/news/turning-waste-from-the-paper-industry-into-beneficial-compounds

This collaboration is between our lab, Maria Paula Robalo (ISEL/IST) and Rita Ventura´s lab at ITQB-NOVA.
Laccases are special enzymes with incredible potential for turning lignin, a major component of plant biomass, into valuable chemicals sustainably. Despite reducing costs over the past two decades, enzymes still represent a significant expense in biorefining processes. Protein engineering can enhance these enzymes' properties and further reduce costs. In our study, we used advanced enzyme engineering tools to increase the efficiency of a hyperthermostable bacterial laccase by over 400 times for a compound related to lignin called 2,6-dimethoxyphenol. The improved enzyme variant also performed better at neutral to alkaline pH levels for various lignin-related compounds. We also optimized the conditions for producing several valuable compounds, such as syringaresinol, dehydrodiconiferyl alcohol, thomasidioic acid, biseugenol, dehydrodiisoeugenol, and diapocynin. Our method offers several advantages: it doesn't use harmful organic solvents, has faster reaction times, requires less enzyme, and delivers excellent yields (up to 100%) compared to other methods. Our biocatalytic system is a significant advancement in lignin chemistry. It enables the efficient production of dimeric compounds used in various biotechnological applications, including medicinal chemistry and polymer synthesis. This progress represents a significant step forward in making the most of lignin, a renewable resource, and contributes to developing sustainable and eco-friendly technologies.
Realizing Lignin's Potential in Biorefining by Bridging Biology, Chemistry, and Engineering
July 14-19, 2024
Lignin, Gordon Research Conference
Stonehill College
320 Washington Street
Easton, Massachusetts, United States
In Lígia´s talk (Enzyme Engineering for the Valorization of Lignin), she showed that we had improved > 400-fold the catalytic efficiency (kcat/Km) of a hyperthermostable bacterial laccase for lignin-related phenolics and that we optimized conditions for the synthesis of lignans and neo-lignans such as syringaresinol, dehydrodiconiferyl alcohol, thomasidioic acid, biseugenol, dehydrodiisoeugenol, and diapocynin, detailing the pH, catalyst concentration, reaction time, temperature, and oxygenation of the reaction mixtures. I show that our biocatalytic system offers several advantages, including being free of organic solvents, achieving faster reaction times, using lower amounts of enzymes and delivering excellent yields (up to 100%) than reported methods in the literature.

We have been at the OXIZYMES meeting again, this time in Lublin, Poland, to present and discuss our latest results with our peers!
Lublin, Poland, 9-12 Jun 2024
We presented three oral presentations (on new carbohydrate oxidases (LO Martins), engineered laccases producing lignans at excellent yields (V Brissos), and immobilized isoeugenol dioxygenases involved in vanillin production ( De Simone)) and one poster presentation. PhD student Carolina F. Rodrigues won the best poster prize for her work on the mechanisms involved in Mn(II) oxidation by bacterial DyP-type oxidases. Awesome!!![]()
![]()


Lígia Martins's journey at a glance is in the GECCO BIOTEC blog:
Groningen, 24 Jun 2024
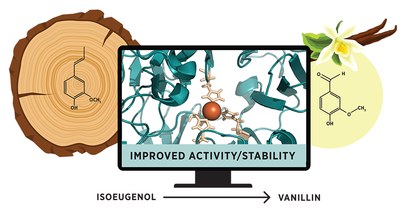
Distal Mutations Enhance Efficiency of Free and Immobilized NOV1 Dioxygenase for Vanillin Synthesis
Oeiras, 13 Jun 2024
https://www.sciencedirect.com/science/article/pii/S0168165624001706?via%3Dihub

NOV1 is a bacterial dioxygenase that holds biotechnological potential by catalyzing the one-step oxidation of the lignin-derived isoeugenol into vanillin, a popular flavoring agent used in food, cleaning products, cosmetics and pharmaceuticals.
This study aims to enhance NOV1 activity and operational stability by identifying distal hotspots located at more than 9 Å from the active site using Zymspot. This tool predicts advantageous distant mutations, streamlining protein engineering. Forty-one variants were constructed using site-directed mutagenesis, and the six most active enzyme variants were then recombined. Two variants, with two and three mutations, showed nearly a 10-fold increase in activity and up to 40-fold higher operational stability than the wild-type.
Furthermore, these variants show 90–100 % immobilization efficiency in metal affinity resins, compared to approximately 60 % for the wild-type. In bioconversions where 50 mM of isoeugenol was added stepwise over 24-h cycles, the 1D2 variant produced approximately 144 mM of vanillin after six reaction cycles, corresponding to around 22 mg, indicating a 35 % molar conversion yield. This output was around 2.5 times higher than that obtained using the wild-type.
Our findings highlight the efficacy of distal protein engineering in enhancing enzyme functions like activity, stability, and metal binding selectivity, thereby fulfilling the criteria for industrial biocatalysts.
Tiago Lopes, a PhD from our lab, attended the MIT Portugal Innovation Workshop 2024 at the Massachusetts Institute of Technology.
Boston, USA, 3-7 Jun 2024
The workshop is designed to bridge the gap between cutting-edge research and real-world applications. It brought together 35 students and researchers, all aspiring or early-stage entrepreneurs, to explore the potential business prospects of their innovative ideas.

Tiago mentions, "As a PhD student specializing in enzyme engineering, this workshop offered invaluable insights into commercializing high-impact research and technologies. The week-long workshop was especially memorable for the enriching conversations with our mentor, Professor Christina Chase. Furthermore, the insights from Nuno Arantes-Oliveira, Marina Hatsopoulos, Isabel Furtado, Dulcie Madden, Elaine Chen, and Gary Schall greatly enhanced the learning experience, offering valuable perspectives on purposeful entrepreneurship. In addition, we had the chance to talk to the Portuguese Minister of Education, Science and Innovation, Fernando Alexandre, and discuss how innovation leads to value-added technology development. Attending the MIT Portugal Innovation Workshop 2024 has been a highlight of my PhD journey. It not only broadened my horizons but also provided a solid base to explore the commercial potential of my research."
Links
Facebook: https://www.facebook.com/MIT.Portugal.Program; Linkedin: https://www.linkedin.com/company/mit-portugal-program/
PT coordination office email: info@mitportugal.org; MIT coordination office email: mitportugal@mit.edu
Marie Skłodowska-Curie Post Doctoral Fellowship
Oeiras, June 2024

Elena Karnišová Potocká was awarded an MSCA postdoctoral fellowship for her multidisciplinary project under the supervision of Rita Ventura (Bioorganic Chemistry) and Lígia O. Martins (Microbial & Enzyme Technology), with the collaboration of Maria Miragaia (Bacterial Evolution and Molecular Epidemiology).
Her project aims to use a toolbox of engineered pyranose and galactose oxidases to generate a library of bioactive compounds as promising candidates with antimicrobial activity and health benefits. Stereospecific enzymatic oxidations combined with chemical reactions will lead to an extensive library of quickly prepared derivatives in only two-step chemoenzymatic processes.
Meeting of the COST Action COZYME Pan-European Network on Computational Redesign of Enzymes
Zagreb, Croatia, 25-26 April 2024 at the Faculty of Science, University of Zagreb, Department of Chemistry organized by Dr. Aleksandra Maršavelski and team.
We had oral sessions of WG 1, WG 2 and WG 3, brainstorming, poster sessions, and a session where researchers who benefit from Short-Term Scientific Missions (STSMs) presented their work. It was an excellent opportunity to strengthen existing networks and create new ones, facilitating knowledge transfer in enzyme engineering using computational and experimental tools.

6th Course Applied Enzymology
6th European Summer School on Industrial Biotechnology
April 8 – 12, 2024 | Groningen | The Netherlands

This course will provide detailed information on the basic principles of enzymes, their engineering, and industrial applications. In addition to expert lectures, the course includes company visits and training using computational enzyme engineering tools.
The course is at the postgraduate level and will be valuable for PhD candidates working with enzymes in academia and industry or considering doing so in biocatalysis, food and feed science, bioprocess engineering, and similar areas.
The graduate school VLAG, research institutes GBB, and ENTEG organize the course.
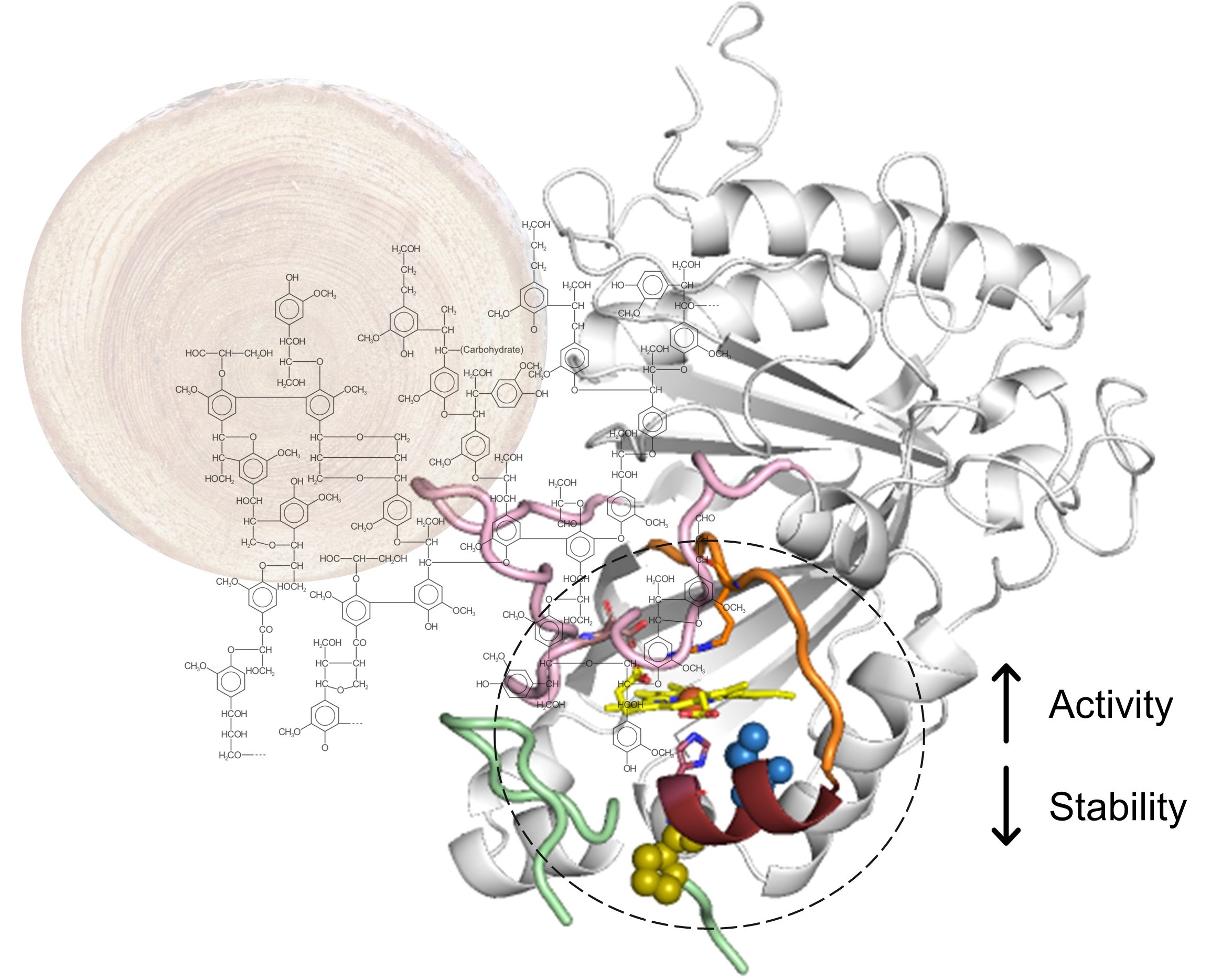
Flexible active‑site loops fine‑tune substrate specificity of hyperthermophilic metallo‑oxidases
Oeiras, 16 Jan 2024
https://link.springer.com/article/10.1007/s00775-023-02040-y
Hyperthermophilic (‘superheat-loving’) archaea found in high-temperature environments such as Pyrobaculum aerophilum contain multicopper oxidases (MCOs) with remarkable efficiency for oxidizing cuprous and ferrous ions. In this work, directed evolution was used to expand the substrate specificity of P. aerophilum McoP for organic substrates. Six rounds of error-prone PCR and DNA shuffling followed by high-throughput screening led to the identification of a hit variant with a 220-fold increased efficiency (kcat/Km) than the wild-type for 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) without compromising its intrinsic activity for metal ions. The X-ray crystal structure analysis reveals four proximal mutations close to the T1Cu active site. One of these mutations is within the 23-residues loop that occludes this site, a distinctive feature of prokaryotic MCOs. The increased flexibility of this loop results in an enlarged tunnel and one additional pocket that facilitates bulky substrate-enzyme interactions. These findings underscore the synergy between mutations that modulate the dynamics of the active-site loop, enabling enhanced catalytic function. This study highlights the potential of targeting loops near the T1Cu for engineering improvements suitable for biotechnological applications.
Highlight in Chemistry Views
Oeiras, 24 Nov 2023
Here is Paulo Durão's latest paper on an environmentally friendly two-step chemo-enzymatic process that effectively transforms the antibiotic rifampicin into non-antimicrobial compounds in ChemBioChem.
Read the highlight here: Chemo-Enzymatic Degradation of Rifampicin
Biocatalysis for biorefineries: The case of dye-decolorizing peroxidases
Biotechnol Adv 2023, 65: 108153
The study of DyP-type peroxidases has significant implications for environmental sustainability and the development of new bio-based products and materials with improved end-of-life options via biodegradation and chemical recyclability, fostering the transition to a sustainable bio-based industry in the circular economy realm.
In this review, we track recent research contributions that furthered our understanding of the activity, stability, and structural properties of DyPs and, particularly, their biotechnological applications.

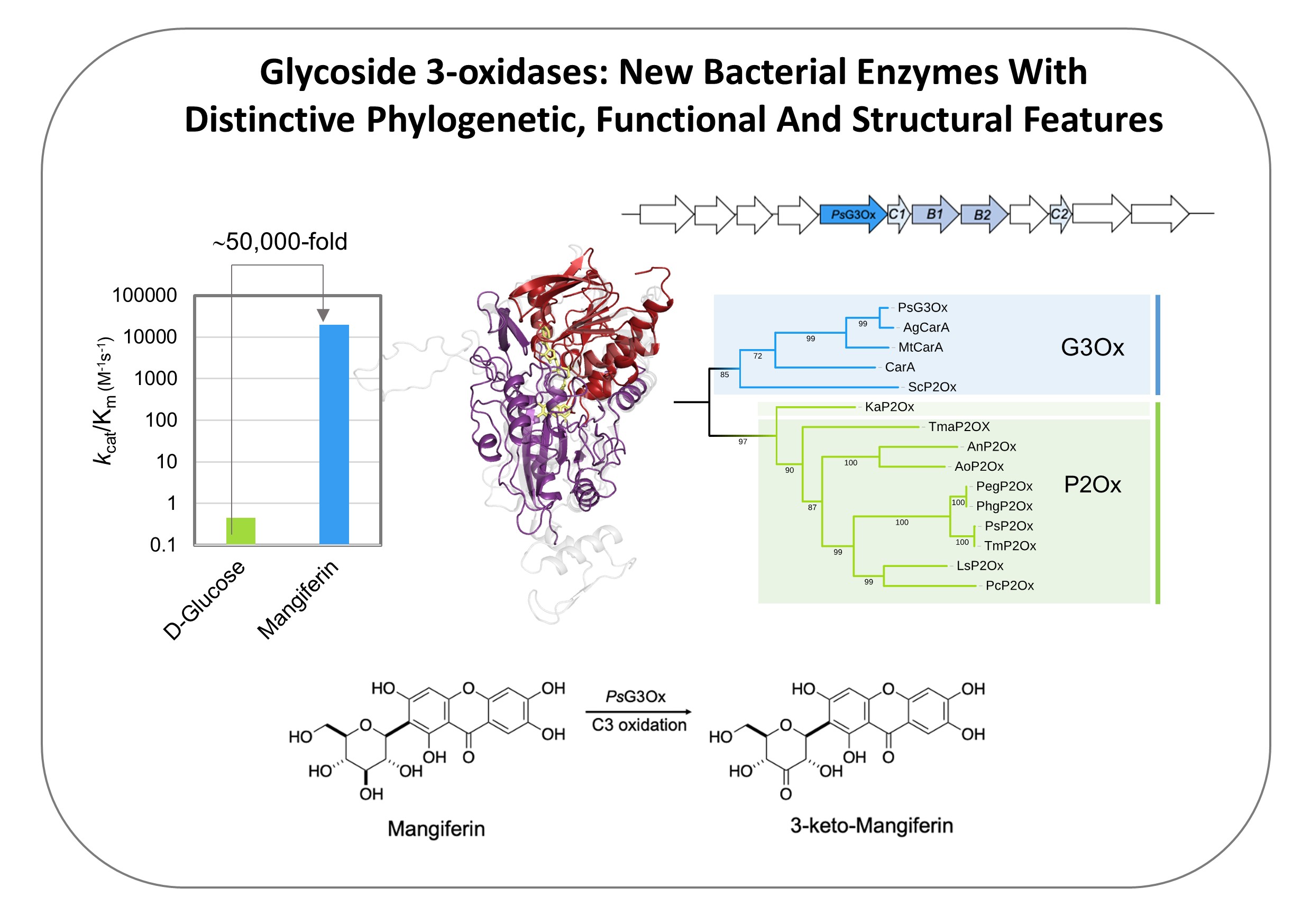
Mechanistic insights into glycoside 3-oxidases involved in C-glycoside metabolism in soil microorganisms
Oeiras, 14 Nov 2023
Check our latest work in Nature Communications that revealed functional and structural details of a critical bacterial catabolic enzyme that oxidizes recalcitrant C-glycosides, abundant and biologically important plant-derived molecules: https://lnkd.in/gc-MqKqA
See ITQB´s highlight: https://www.itqb.unl.pt/news/breaking-down-bacterial-strategies-for-complex-sugar-degradation
- We are happy that the first four (4!) authors, André Taborda, Tomás Frazão, Miguel Rodrigues, and Xavier Fernandez-Luengo, are PhD students, marking a great career start.
- The work was a collaborative effort involving three (3!) different research groups at ITQB Universidade Nova de Lisboa, specializing in Enzymology, X-ray crystallography, and Organic Chemistry.
- Additionally, we had the opportunity to collaborate with colleagues from Universitat Autònoma de Barcelona, Universidade do Algarve, and Zymvol Biomodeling, a company based in Barcelona, Spain, fostering an academia-private sector partnership.
- Last but not least, we've achieved a commendable gender balance in this endeavor!
We have been at the Gordon Research Conference on Carbohydrate-Active
Enzymes for Glycan Conversions held 07/23/2023 - 07/28/2023 at Proctor Academy in Andover, New Hampshire, US!

MET Lab at BIOTRANS 2023
La Rochelle, France, 25-28 June 2023
BIOTRANS 2023 provides an overview of the latest advances in biocatalysis and biotransformations, gathering innovative and interdisciplinary strategies to overcome scientific and technological hurdles. Various topics will be covered, such as enzyme discovery and design, reaction engineering, enzyme mechanisms, computational methods, synthetic biology, metabolic engineering, (chemo)enzymatic cascades, and industrial biocatalysis.

MET Lab at NOVEL ENZYMES 2023
Greifswald, Germany, 28-31 March 2023
The 7th International Conference on Novel Enzymes covered the most recent and exciting advances in Biocatalysis. This field has seen tremendous development—especially in the past few years—with major achievements in areas such as enzyme discovery, advanced computational tools for enzyme engineering, novel chemistry catalyzed by engineered biocatalysts, integration of several enzymes into multistep cascades, and combinations with metal, organo-, photo-, and electro-chemistry.
André Taborda, Carolina F Rodrigues, and Mario De Simone have presented their work on studies targeting the investigation and engineering of Glycoside 3-2-oxidase, DyP-type peroxidases, and Iron monooxygenases!
The website of COST Action CA21162 - COZYME: Establishing a Pan-European Network on Computational Redesign of Enzymes (2022-26) has been published!
March 1, 2023
Lígia O Martins is the leader of Work Group 3 (Experimental Evaluation and Characterization of Enzymes).
The website contains relevant information on how COZYME COST Action can support short-term scientific stays, attendance at conferences, and other activities that will be organized in the future.
Follow the COZYME LinkedIn page for updates.
MET Lab at Beyond the Lab Course
Computing and business skills for science students
February 20-24, 2023, Barcelona, SP
Zymvol organized the course in collaboration with the B-LigZymes (GA824027) and BioInspireSensing European projects. It was an exciting first edition, with a program where computing & business were as entangled as innovation requires nowadays 💡
We dived head-first into:
🔹 Homology modeling & Alphafold
🔹 IT & data tools for business
🔹 Branding & Storytelling
🔹 Business models & Design thinking
🔹 Public & Private Fundraising
🔹 IP protection
🔹 Fireprot for Protein Engineering
Carolina Dias worked at METGEN company for one year in the Netherlands and Finland. She worked on molecular biology to improve fungal strains' degradative machinery, which can decompose the often heterogeneous substrates available and valorize lignocellulose components. Later, she worked on enzymatic bioprocesses.
This stay was funded by the H2020 RISE B-Ligzymes project (GA824027).
Sittard-Geleen, Netherlands (Nov 22-May 23) and Kaarina, Finland (July-Dec 23)

Patrícia Borges at Zymvol Biomodeling used molecular dynamic analysis, substrate docking, calculation of electron transfer pathways, and analysis of protein residue networks to reveal the role of mutations of an evolved variant of the multicopper oxidase McoA from Aquifex aeolicus.
This stay was funded by the H2020 RISE B-Ligzymes project (GA824027).
Barcelona, Spain, Oct-Dec 2022

During his training at Zymvol Biomodeling, Tomás Frazão studied the catalytic mechanism of G3Ox from P. siccitolerans using MDs.
This stay was funded by the H2020 RISE B-Ligzymes project (GA824027).
Barcelona, Dec 2021-July 2022

João Costa during his training period at GECCO BIOTECH! He learned and practiced modern molecular biology tools and gained experience in enzyme expression optimization and purification methods at different scales in a small, highly dynamic company embedded in the campus of the University of Groningen.
This stay was funded by the H2020 RISE B-Ligzymes project (GA824027).
Groningen, The Netherlands, Dec 2021-Apr 2022

A Wide Array of Lignin-Related Phenolics are Oxidized by an Evolved bacterial Dye-decolourising Peroxidase
Oeiras, 21.12.2022
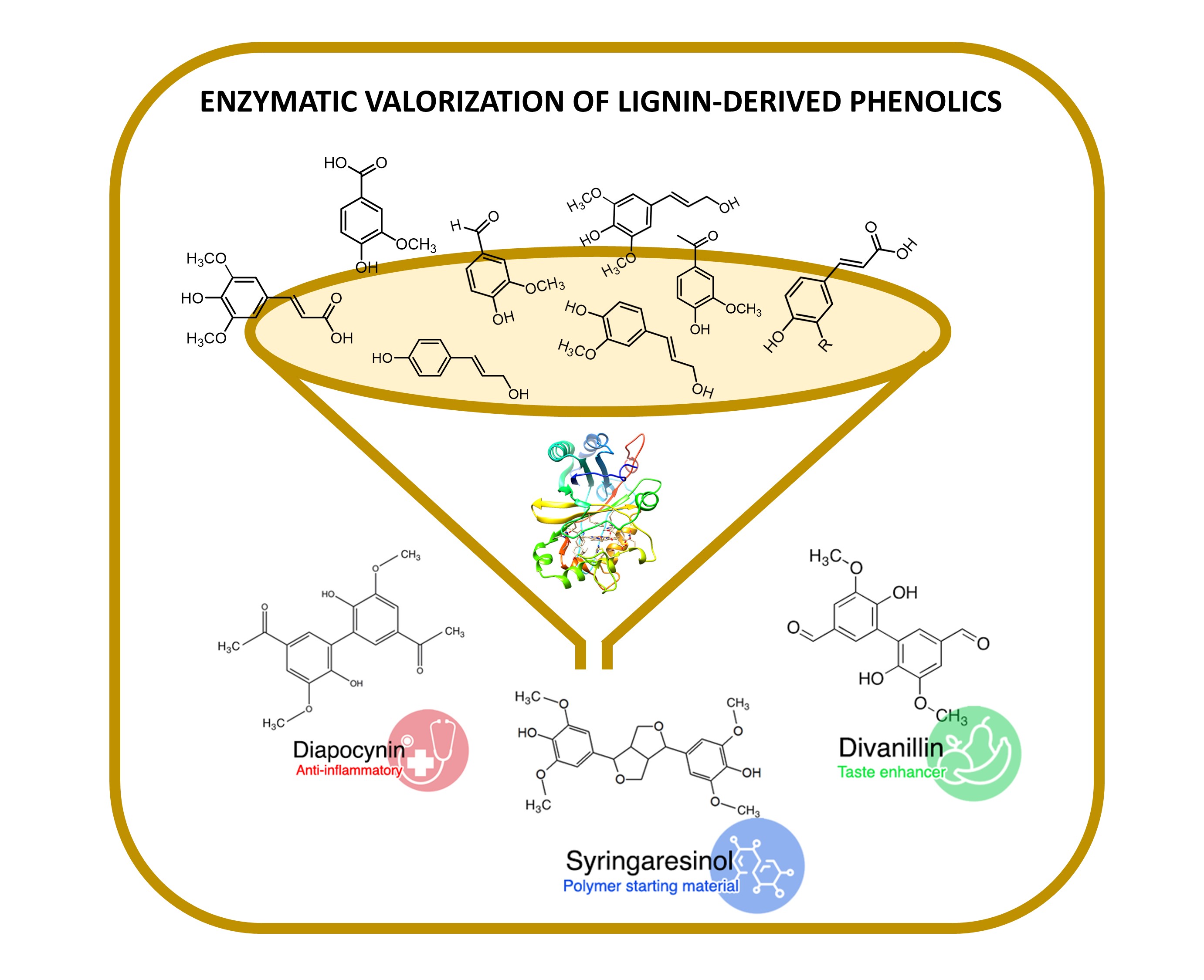
Lignin is the second most abundant natural polymer next to cellulose and by far the largest renewable source of aromatic compounds on the planet. Dye-decolorising peroxidases (DyPs) are biocatalysts with immense potential in lignocellulose biorefineries to valorize emerging lignin building blocks for environmentally friendly chemicals and materials. This work investigates the catalytic potential of the engineered PpDyP variant 6E10 for the oxidation of 24 syringyl, guaiacyl, and hydroxybenzene lignin-phenolic derivatives. Variant 6E10 exhibited up to 100-fold higher oxidation rates at pH 8 for all the tested phenolic substrates than the wild-type enzyme and other acidic DyPs described in the literature. The main products of the reactions were dimeric isomers with molecular weights of (2 × MWsubstrate - 2H). Their structure depends on the substitution pattern of the aromatic ring of substrates, i.e., of the coupling possibilities of the primarily formed radicals upon enzymatic oxidation. Among the dimers identified were syringaresinol, divanillin, and diapocynin, important sources of structural scaffolds exploitable in medicinal chemistry, food additives, and polymers.
Read the paper here: https://www.sciencedirect.com/science/article/pii/S187167842200067X
Diogo Silva, our next Ph.D. holder at the Lab:), attended the 8th International Summer School in Technology Transfer in Life Sciences.
Dresden, Germany, 26-30th September 2022
Diogo learned how to identify innovative potential within his research, learn about the options for bringing inventions into the market and generating money with them, develop his idea into a real innovation with the help of experts, gain knowledge on potential funding opportunities, and connect with successful founders of life science companies to learn about the challenges and solutions. It was a gratifying experience!

Unveiling Molecular Details behind Improved Activity at Neutral to Alkaline pH of an Engineered DyP-type Peroxidase
Oeiras, 26.7.2022
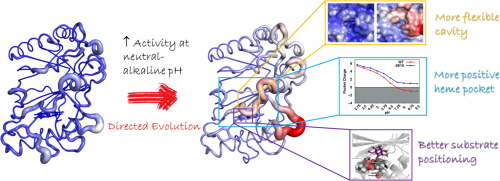
Collaboration with Tomás Silva and Miguel Machuqueiro (Faculdade Ciências da UL), Eduardo Melo (Univ do Algarve), Laura Masgrau, Marina Canellas and Maria Fatima Lucas (Zymvol Biomodeling, SP).
This work obtained high-resolution PpDyP wild-type structures and two engineered variants (6E10 and 29E4), generated by directed evolution. The X-ray crystal structures revealed the typical ferredoxin-like folds, with three heme access pathways, two tunnels, and one cavity, limited by three long loops, including catalytic residues. Variant 6E10 displays significantly increased loops’ flexibility that favors function over stability: despite the considerably higher catalytic efficiency, this variant shows poorer protein stability than wild-type and 29E4 variants. Constant-pH MD simulations revealed a more positively charged microenvironment near the heme pocket of variant 6E10, particularly in the neutral to alkaline pH range. This microenvironment affects enzyme activity by modulating the pKa of essential residues in the heme vicinity. It should account for variant 6E10 improved activity at pH 7–8 compared to the wild-type and 29E4, which show optimal enzymatic activity close to pH 4. Our findings shed light on the structure-function relationships of DyPs at the molecular level, including their pH-dependent conformational plasticity. These are essential for understanding and engineering the catalytic properties of DyPs for future biotechnological applications.
Read the paper here: https://www.sciencedirect.com/science/article/pii/S2001037022003129
MET Lab @ OXIZYMES2022
Siena, Italia, 4-8 July 2022
OxiZymes 2022 emphasized the discovery of new oxidoreductases and the latest advances in their biotechnological applications. The conference format was highly interactive, with ample time for stimulating discussions, interdisciplinary interactions, and social meetings alongside the scientific sessions. André Taborda, Carolina Dias, Carolina F Rodrigues, Magalí Socozza, Lígia Martins, Mario De Simone, Patrícia Tavares, and Vânia Brissos have presented their work on studies targeting Pyranose 2-oxidase, Galactose Oxidase, DyP-type peroxidases, Iron monooxygenases, and hyperthermophilic Laccases!
http://www.congressi.unisi.it/oxizymes22/

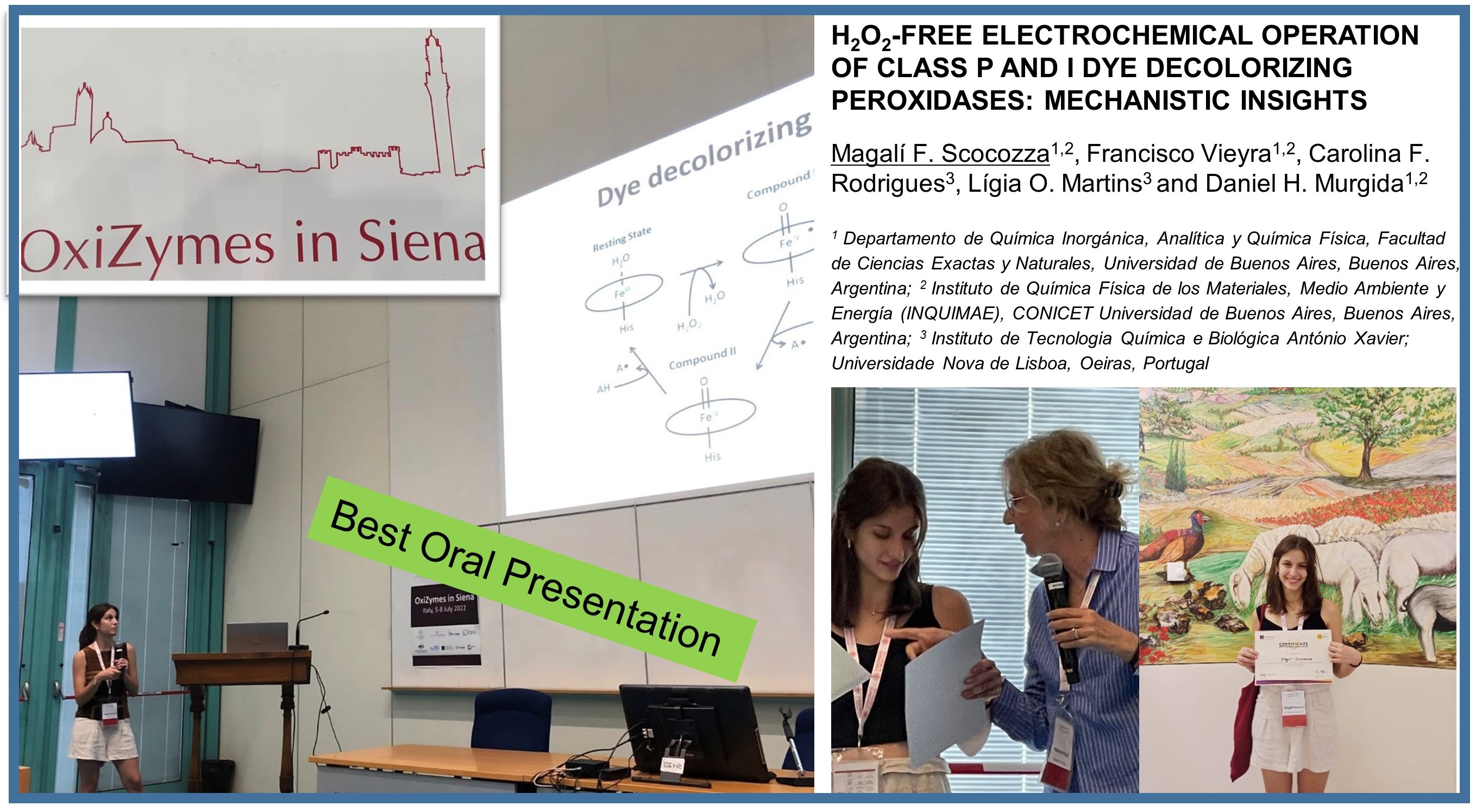
Magalí Scocozza (Ph.D. student co-supervised by Daniel Murgida and Lígia Martins) won the best oral presentation!! 
"Girls should never be afraid to be smart."
Rationally Guided Improvement of NOV1 Dioxygenase for the Conversion of Lignin-Derived Isoeugenol to Vanillin
Oeiras, 13.6.2022
Collaboration with Andrea Mattevi (Univ of Pavia, IT), Emanuele Monza, Lur Alfonso, and Maria Fatima Lucas (Zymvol Biomodeling, SP).
NOV1 is a dioxygenase that catalyzes the one-step, coenzyme-free oxidation of isoeugenol into vanillin and holds enormous biotechnological potential for the complete valorization of lignin as a sustainable starting material for biobased chemicals, polymers, and materials. In this study, 35 variants were designed based on the structural analysis of the NOV1 enzyme, in silico dockings, comparative structural alignments, and Rosetta computation-based design, constructed and examined for activity toward the isoeugenol substrate. The S283F variant emerged as the most active and stable variant and a promising biocatalyst for vanillin production. Furthermore, a combination of kinetics, stability measurements, X-ray diffraction, and molecular dynamics allowed the identification of the molecular basis behind the improved properties of the S283F variant.
Read the paper here: https://pubs.acs.org/doi/pdf/10.1021/acs.biochem.2c00168
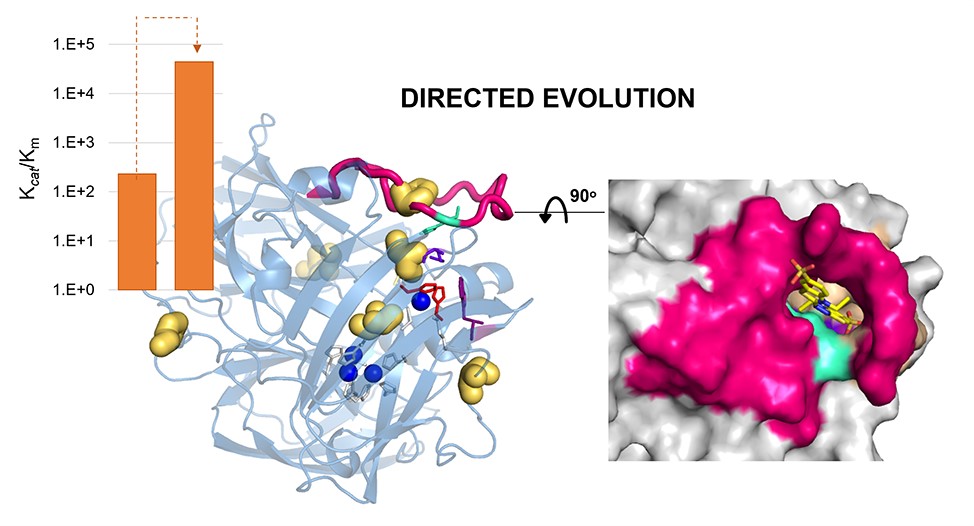
Distal Mutations Shape Substrate-Binding Sites during Evolution of a Metallo-Oxidase into a Laccase
Oeiras, 19.4.2022

Collaboration with Tiago Cordeiro (Dynamic Structural Biology Lab, ITQB), Carlos Frazão (Structural Biology Lab, ITQB), Emanuele Monza, Reyes Nunez, Laura Masgrau, and Maria Fatima Lucas (Zymvol Biomodeling, SP).
Laccases are in increasing demand as innovative solutions in the biorefinery fields. In this work, we combine mutagenesis with structural, kinetic, and in silico analyses to characterize the molecular features that cause the evolution of a hyperthermostable metallo-oxidase from the multicopper oxidase family into a laccase (kcat 273 s−1 for a bulky aromatic substrate). We show that six mutations scattered across the enzyme collectively modulate dynamics to improve the binding and catalysis of a bulky aromatic substrate. Replacing residues during the early stages of evolution is a stepping stone for altering the shape and size of substrate-binding sites. Binding sites are then fine-tuned through high-order epistasis interactions by inserting distal mutations during later stages of evolution. Allosterically coupled, long-range dynamic networks favor catalytically competent conformational states more suitable for recognizing and stabilizing the aromatic substrate. This work provides mechanistic insight into enzymatic and evolutionary molecular mechanisms and spots the importance of iterative experimental and computational analyses to understand local-to-global changes.
Read the paper here: https://pubs.acs.org/doi/pdf/10.1021/acscatal.2c00336
Check the highlight: https://www.itqb.unl.pt/news/evolving-towards-a-greener-and-better-tomorrow
At the news!!!

Loops with a role in catalysis in Dye-decolorising peroxidases
Oeiras, 12.10.2021
Check out our new publication, in which a combination of protein engineering and structural analysis contributed to a better understanding of the role of loops close to the heme pocket in catalysis.
"It was shown that loops around the heme pocket in DyPs account for local flexibility, which is critical for modulating important enzyme properties such as activity, specificity, and stability. The obtained results suggest that tuning the dynamics of catalytically relevant loops is possible, which might be essential for improving or emerging new catalytic properties in these enzymes."
Read the paper here: https://www.mdpi.com/1422-0067/22/19/1086
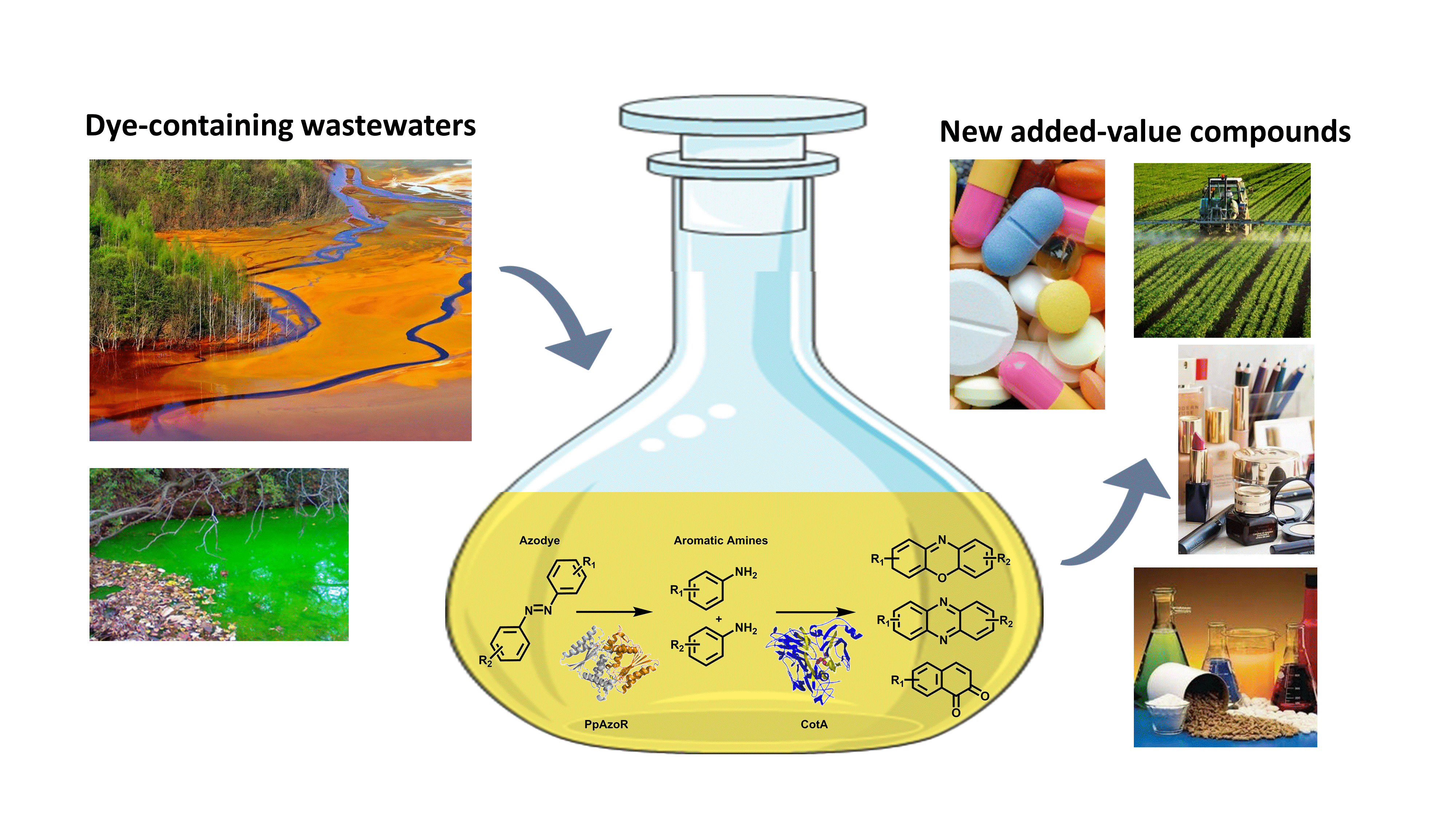
Wasteful Azo Dyes as a Source of Biologically Active Buiding Blocks
Oeiras, 15.06.2021
This work developed an environment-friendly enzymatic strategy to valorize dye-containing wastewaters into valuable aromatic compounds: aromatic amines, phenoxazinones, and phenazines naphthoquinones.
Read the full paper here: https://www.frontiersin.org/articles/10.3389/fbioe.2021.672436/full
Check our new video that describes the activities of our laboratory:
https://youtu.be/ksLWrPGHxk0?list=PLlGQ36SFjxrWcDyZav3UI2XbxFmlwO5h9
and what about our group picture "into the wild"-style?:)

@spontaneousrita (IG)
Our paper on the synthesis of 4‐arylamino‐1,2‐naphthoquinones in collaboration with Maria Paula Robalo made the cover of Advanced Synthesis & Catalysis.
Oeiras, 19.08.2020
Diogo Batuca/Estúdio João Campos created the front cover picture and illustrated an eco‐friendly approach for the one‐pot enzymatic synthesis of 4‐arylamino‐1,2‐naphthoquinones using CotA‐laccase.

The distinct specificity of laccase towards oxidizable amines allows the design of new frameworks, broadening the competence of laccases as biocatalysts and opening new perspectives for their use in organic synthesis.
A biocatalytic process is a green approach with short reaction times, mild conditions, an aqueous medium & room temperature.
See the full paper: dx.doi.org/10.1002/adsc.20200000
ITQB NOVA researchers have unraveled structural details related to multicopper oxidases catalysis
Oeiras, 2.06.2020
 The study was published in ACS Catalysis and resulted from the collaboration between three ITQB NOVA labs: the Microbial and Enzyme Technology Lab, led by Lígia Martins; the Dynamic Structural Biology Lab, led by Tiago N. Cordeiro; and the Structural Biology Lab, led by Carlos Frazão. This in-house synergy allowed unprecedented access to laccases’ structural aspects related to catalysis. This research also included molecular dynamic simulations, done by Zymvol Biomodeling.
The study was published in ACS Catalysis and resulted from the collaboration between three ITQB NOVA labs: the Microbial and Enzyme Technology Lab, led by Lígia Martins; the Dynamic Structural Biology Lab, led by Tiago N. Cordeiro; and the Structural Biology Lab, led by Carlos Frazão. This in-house synergy allowed unprecedented access to laccases’ structural aspects related to catalysis. This research also included molecular dynamic simulations, done by Zymvol Biomodeling.
Loop opening and closure are essential to hang and stabilize substrates, setting up the active site for efficient catalysis. Our results indicate that the Met-loop and its dynamics display key regulatory functions capable of controlling the access of substrates and determining the chemical environment of the metallo-oxidase McoA from Aquifex aeolicus. “This has fundamental implications in identifying elements with a role in discerning substrate specificity and catalytic rates among multicopper oxidases. These motifs are also important targets of engineering for the development of industrial and biomedical applications", highlights Lígia O. Martins.
The ITQB NOVA researchers will continue to pursue this path using high-resolution structural studies with nuclear magnetic resonance, combined with molecular dynamics simulations, to provide new insights into Met-rich element's dynamic conformational landscape and substrate binding.
The work was performed within the frame of FCT funded project FitZymes (PTDC/BII-BBF/29564/2017) and the B-LigZymes project from the European Union’s Horizon 2020 Research and Innovation Programme, both coordinated by Lígia Martins and developed in partnership with the teams led by Carlos Frazão and Tiago Cordeiro, from ITQB NOVA, and Emanuele Monza and Maria Fátima Lucas, at Zymvol Biomodeling in Barcelona.
ITQB NOVA researchers - Lígia Martins, Vânia Brissos, Carlos Frazão, Patrícia Borges, Tiago Cordeiro and Guillem Hernandez.
ACS Catalysis | doi.org/10.1021/acscatal.0c01623
Methionine-Rich Loop of Multicopper Oxidase McoA Follows Open-to-Close Transitions with a Role in Enzyme Catalysis Patrícia T. Borges, Vânia Brissos, Guillem Hernandez, Laura Masgrau, Maria Fátima Lucas, Emanuele Monza, Carlos Frazão*, Tiago N. Cordeiro*, and Lígia O. Martins*
MET Lab at Microbiotech19
Coimbra, 5-7 Dec 2019
Although directed at Portuguese scientists, this biennale conference aims to provide an international scientific forum where researchers from all over the world share their knowledge, exchange ideas, and discuss scientific policy and regulatory issues associated with microbiology and biotechnology in the future.
MET Lab at Biotrans19, the 14th International Symposium on Biocatalysis and Biotransformations
Groningen, 7-11 July 2019

The BioTrans 2019 symposium showcased the most recent advances in biocatalysis research, covering various cutting-edge topics, from reprogramming synthetic biology and redesigning natural enzymes to developing new (chemo)enzymatic cascades and novel classes of artificial enzymes. The symposium included invited lectures from experimentalists and theoreticians from both academia and industry. The invited speakers were all current or emerging leaders in their respective sub-fields, bringing together the chemistry and biology interface. Combining these speakers in one symposium provided an exceptional opportunity to bridge disciplines and engage in active discussion while maintaining a solid link to academic and industrial applications.
Kick-off meeting of the SMARTBOX project
Desteldonk, Belgium, 9-10.05.2019
https://www.bbi-europe.eu/projects/smartbox
https://www.smartbox-project.eu/
The main objective of SMARTBOX is to enable biobased industries to use cheap, robust, and efficient oxidative e nzymes, which can be readily engineered towards industry requirements. To achieve this, SMARTBOX will develop an advanced computational engineering platform specifically for oxidative enzymes, which can automatically screen for improved enzyme variants with minimal human intervention. Through machine learning, the computational screening algorithms will be trained with experimental screening results obtained from mutant screening on real feedstocks. The algorithms will consequently be adapted towards feedstock type and quality. At the same time, the time and costs associated with oxidative enzyme engineering will be reduced 10-fold compared to state-of-the-art directed evolution strategies and 5-fold compared to SOTA computational methods, lowering the threshold for the biobased industries to implement oxidative biocatalysis. As oxidative enzymes have the potential to make several (bio)chemical processes more environmentally friendly (milder reaction conditions, no side-product formation, etc.), this development is expected to have a significant economic and environmental impact in the long term.
nzymes, which can be readily engineered towards industry requirements. To achieve this, SMARTBOX will develop an advanced computational engineering platform specifically for oxidative enzymes, which can automatically screen for improved enzyme variants with minimal human intervention. Through machine learning, the computational screening algorithms will be trained with experimental screening results obtained from mutant screening on real feedstocks. The algorithms will consequently be adapted towards feedstock type and quality. At the same time, the time and costs associated with oxidative enzyme engineering will be reduced 10-fold compared to state-of-the-art directed evolution strategies and 5-fold compared to SOTA computational methods, lowering the threshold for the biobased industries to implement oxidative biocatalysis. As oxidative enzymes have the potential to make several (bio)chemical processes more environmentally friendly (milder reaction conditions, no side-product formation, etc.), this development is expected to have a significant economic and environmental impact in the long term.
MET lab (ITQB) is involved in WP3- Enzyme Platform Development
New EU project on bacterial enzymes and bioprocesses for lignin valorization
Biorefineries for the XXI century
Oeiras, 30.11.2018
B-LigZymes is an EU-funded RISE project, a consortium coordinated by ITQB NOVA with seven academic organizations and three companies to address current limitations in lignocellulose degradation. The aim is to generate eco-friendly technological and economical solutions inspired by fundamental research, leading to an increasingly knowledge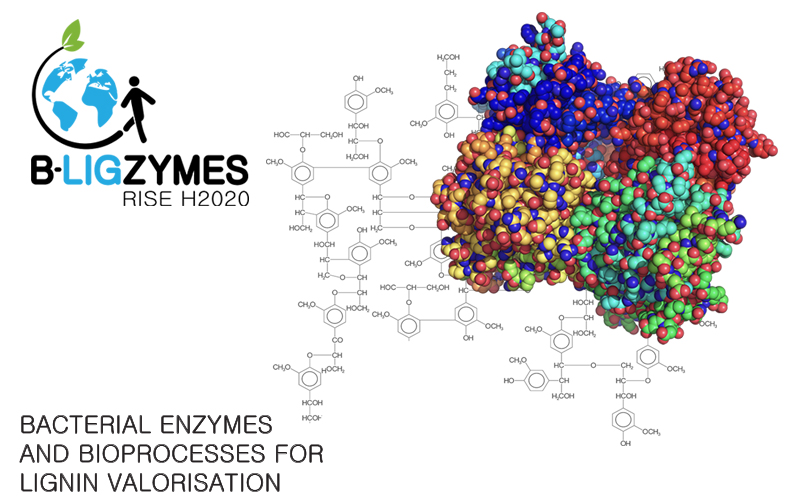 -based, inclusive, circular economy. The project, now signed, will officially start in February 2019 and is endowed with 1 million euros for training and staff exchange for the next four years.
-based, inclusive, circular economy. The project, now signed, will officially start in February 2019 and is endowed with 1 million euros for training and staff exchange for the next four years.
The industrial exploitation of plant biomass is a promising alternative source of renewable chemicals, materials, energy, and fuels for future sustainable development. Lignin is Earth's most abundant aromatic polymer and the second most abundant raw material next to cellulose. Still, it is currently considered a bio-waste by lignocellulose industries due to its resistance to degradation, which makes it very difficult and expensive to reuse. Working on new and eco-friendly ways to break it down into its components could open the possibility of developing lignin-based products - the market for these products is estimated to be around 12 billion € by 2020-2025, with lignin-based phenols and carbon fibers poised to capture the largest market potential.
 "B-LigZymes relies on a holistic approach for developing sustainable biocatalytic processes based in bacterial systems largely unexplored but potentially rich in new ligninolytic enzymes for biotech use. B-LigZymes goals are to identify and isolate new bacterial ligninolytic enzymes, improve their performance and robustness by relying on iterative experimental and computational protein engineering tools, and set up enzymatic processes for lignin depolymerization and fractionation into a phenolics platform for environmental friendly breakthrough applications", according to Lígia O Martins, coordinator of the B-LigZymes. "We have put together an international, interdisciplinary, and intersectoral consortium, and we look forward to presenting some results of this melting pot during the next four years." The existent complementarity among partners enables bi-directional international and intersectoral staff exchanges and the sharing of knowledge and ideas from research to market.
"B-LigZymes relies on a holistic approach for developing sustainable biocatalytic processes based in bacterial systems largely unexplored but potentially rich in new ligninolytic enzymes for biotech use. B-LigZymes goals are to identify and isolate new bacterial ligninolytic enzymes, improve their performance and robustness by relying on iterative experimental and computational protein engineering tools, and set up enzymatic processes for lignin depolymerization and fractionation into a phenolics platform for environmental friendly breakthrough applications", according to Lígia O Martins, coordinator of the B-LigZymes. "We have put together an international, interdisciplinary, and intersectoral consortium, and we look forward to presenting some results of this melting pot during the next four years." The existent complementarity among partners enables bi-directional international and intersectoral staff exchanges and the sharing of knowledge and ideas from research to market.
ITQB NOVA team includes Lígia O Martins (coordinator of B-Ligzymes) and researchers Smilja Todorovic (Raman BioSpectroscopy Lab), Vânia Brissos (Microbial and Enzyme Technology), Tiago Cordeiro (Dynamic Structural Biology Lab), and Carlos Frazão (Structural Biology Lab).
Diana Santos awarded Best Short Talk |
Oeiras, 4.07.2018
The short talk “Improving a bacterial pyranose 2-oxidase using a combination of rational design and directed evolution for biosensor applications,” presented by Diana Santos, research student at the Microbial and Enzyme Technology Laboratory at ITQB NOVA, was awarded the INOFEA Early Career Award For Applied Biocatalysis Or Nanobiotechnology for Best Short Talk at the 18th European Congress On Biotechnology, which took place in Geneva, Switzerland 1-4 July.
The European Federation of Biotechnology is a non-profit federation of National Biotechnology Associations, Learned Societies, Universities, Scientific Institute s, Biotech Companies, and individual biotechnologists working to promote biotechnology throughout Europe and beyond. EFB promotes the safe, sustainable, and beneficial use of fundamental research and innovation in life sciences while providing a forum for interdisciplinary and international cooperation. INOFEA awarded two cash prizes and free registration at the next Congress to the early career scientists who presented the best short talk and poster on topics related to Nanobiotechnology or Applied Biocatalysis during the Congress.
s, Biotech Companies, and individual biotechnologists working to promote biotechnology throughout Europe and beyond. EFB promotes the safe, sustainable, and beneficial use of fundamental research and innovation in life sciences while providing a forum for interdisciplinary and international cooperation. INOFEA awarded two cash prizes and free registration at the next Congress to the early career scientists who presented the best short talk and poster on topics related to Nanobiotechnology or Applied Biocatalysis during the Congress.
Improving a bacterial pyranose 2-oxidase using rational design and directed evolution for biosensor applications. Diana Santos1, Sónia Mendes1, Vânia Brissos1, Willem J.H van Berkel2, Lígia O. Martins1, 1Instituto de Tecnologia Química e Biológica António Xavier, Universidade Nova de Lisboa, 2Laboratory of Biochemistry, Wageningen University, The Netherlands
Summer Science @ITQB NOVA project selected for Microbiotech17
Oeiras, 12.11.2017
Summer Science @ ITQB NOVA is a Summer course designed to provide undergraduate students the opportunity to experience science in a cutting-edge research institute. Starting in 2016, it puts students for one week in a laboratory of their choice to develop a project under the supervision of a senior researcher.
João Zagalo Pereira was one of the selected students for the class of 2017, and the work he developed at Lígia Martins Lab with postdocs Vânia Brisso s and Sónia Mendes was one of the selected oral presentations for MICROBIOTEC’17, the joint congress of the Portuguese Society of Microbiology and the Portuguese Society of Biotechnology. “Comparison of the activity of Pseudomonas putida PpDyP peroxidase and its evolved variant 6E10 for 28 lignin-related phenolics” was included in the symposium “Industrial and Food Microbiology and Biotechnology”.
s and Sónia Mendes was one of the selected oral presentations for MICROBIOTEC’17, the joint congress of the Portuguese Society of Microbiology and the Portuguese Society of Biotechnology. “Comparison of the activity of Pseudomonas putida PpDyP peroxidase and its evolved variant 6E10 for 28 lignin-related phenolics” was included in the symposium “Industrial and Food Microbiology and Biotechnology”.
“This project is part of our ongoing work on improving the properties of bacterial enzymes for lignin degradation and valorization. João Zagalo had very good results during his period as a Summer Science student at our lab, and we encouraged him to submit an abstract to Microbiotech17”, said Lígia Martins, responsible investigator. “It was a pleasure, as a supervisor and a professor, to see him do an oral presentation of this project. We are always available to welcome and nourish motivated young students, and João Zagalo did a great job that was recognized this way”.
Lígia Martins Lab’s work distinguished by the American Chemical Society |
ITQB NOVA research on the spotlight
Oeiras, 17.10.2017
The American Chemical Society publications have chosen a recent paper by Lígia Martins Lab as one of the 20 promising works published in their journals. “ACS Select” collection highlights recent publications from the Journal of the American Chemical Society, ACS Catalysis, and Inorganic Chemistry that showcase the latest groundbreaking research in this field. According to ACS, the 20 selected articles “illustrate the great promise of biomolecular catalysts and the diverse range of applications and technologies in this flourishing area of chemistry.”
Lígia Martins's Lab's work on improving the properties of a bacterial enzyme for lignin degradation and valorization was one of the selected papers. As stated by ACS Select:
Congratulations all.
Original article
ACS Catal. 2017, 7, 3454−3465 DOI 10.1021/acscatal.6b03331
Engineering a Bacterial DyP-Type Peroxidase for Enhanced Oxidation of Lignin-Related Phenolics at Alkaline pH. Vânia Brissos, Diogo Tavares, Ana Catarina Sousa, Maria Paula Robalo and Lígia O. Martins
Evolution-in-a-test-tube |
Engineering a bacterial enzyme to degrade natural raw material
Oeiras, 19.04.2017
Lignin is the most abundant aromatic natural polymer on Earth and can be a key renewable source of high-value chemicals, materials, and fuel precursors. Millions of tonnes of lignin preparations are produced by the paper industry every year, but lignin is currently considered a biowaste because of its inherent heterogeneity and recalcitrance to degradation. Finding optimized and environment-friendly biological routes for lignin valorization have been the focus of intensive research. Indeed for most industrial process applications, the conditions are so far different from those found in nature that organisms and their enzymes must be modified to achieve the necessary properties. The development of laboratory engineering strategies has been followed in Lígia Martins Lab. Their most recent results in collaboration with a group from ISEL/Técnico have just been published at ACS Catalysis.
ITQB NOVA researchers used directed evolution approaches as an alternative to rationally designing modified proteins on a bacterial Pseudomonas putida DyP-type peroxidase. After only three rounds of random mutagenesis and screening, the resulting variant enzyme showed 100 times more activity towards the oxidation of phenolic and aromatic amines, lignin model compounds, and Kraft lignin itself, with an optimal pH, shifted to alkaline values, resistance to hydrogen peroxide inhibition and enhanced production yields, providing clear advantages for application in lignocellulose bioprocesses. The obtained evolved variants were thoroughly characterized, and the role of acquired mutations was unveiled from the catalytic, stability, and structural viewpoints.
“The idea of “made-to-order” enzymes is both fascinating and challenging, and it can serve various purposes in different fields. This is possible through the application of directed evolution which mimics the natural evolution cycle in a laboratory setting, not an easy route but certainly the most effective and exciting approach to engineer enzymes”, said Lígia Martins, the main author of the work. “We will continue to explore the potential of engineering enzymes for different purposes, and look forward to seeing the applications and the fundamental knowledge derived”.
Original article
ACS Catal. 2017, 7, 3454−3465 DOI 10.1021/acscatal.6b03331
Engineering a Bacterial DyP-Type Peroxidase for Enhanced Oxidation of Lignin-Related Phenolics at Alkaline pH. Vânia Brissos, Diogo Tavares, Ana Catarina Sousa, Maria Paula Robalo and Lígia O. Martins
ITQB e iBET no Festival Nacional de Biotecnologia | Pavilhão do Conhecimento e Mercado da Ribeira acolhem investigadores
Nos próximos dias 10, 11 e 12 de Abril, o ITQB e o IBET estarão presentes no Festival Nacional de Biotecnologia. Durantes estes dias, investigadores e especialistas de 28 instituições científicas e empresas da área da Biotecnologia apresentam actividades para o público no Pavilhão do Conhecimento (10 e 11 de Abril) e na Time Out - Mercado da Ribeira (12 de Abril).
As actividades preparadas para o Festival Nacional de Biotecnologia mostram uma pequena parte das actividades de investigação do ITQB e do iBET, com aplicações na saúde, no ambiente e na agricultura. Os projectos escolhidos para este evento incluem a utilização de enzimas para a degradação de poluentes, o uso de esporos bacterianos como probióticos, a produção de fármacos em células de plantas, as novas ferramentas disponíveis para o melhoramento de plantas, a tecnologia de células animais, a purificação de biofarmacos e a cristalografia.
Como é habitual nas actividades de divulgação do ITQB, serão os próprios investigadores que ajudarão os visitantes a conhecer um pouco melhor a investigação levada a cabo no instituto. Desta vez, teremos também a ajuda de dois jovens embaixadores da biotecnologia, alunos do ensino secundário integrados no projecto internacional World Biotech Tour e que contam com investigadores do ITQB e iBET como mentores.
O Festival Nacional de Biotecnologia é uma iniciativa da World Biotech Tour (na qual o ITQB participa) coordenada pela Association of Science-Technology Centers, com o apoio da Biogen Foundation. O Pavilhão do Conhecimento é o primeiro centro de ciência a receber este projecto.
Associado ao Festival, na quinta-feira, dia 9 de Abril pelas 18h00, a Ciência Viva promove também o 9º Café de Ciência no Parlamento, desta vez dedicado à Biotecnologia e que conta também com a presença de investigadores do ITQB.
E o Festival Nacional de Biotecnologia foi assim:
Biotecnologia para uma economia circular
Este projecto tem como objectivo seleccionar, caracterizar e melhorar microrganismos e enzimas capazes de degradar corantes sintéticos encontrados nos efluentes de várias indústrias, nomeadamente na têxtil. Além das vantagens para o ambiente, esta biodegradação / transformação pode dar origem a novas moléculas biologicamente activas, tais como antibióticos, agentes antibacterianos, anticancerígenos, pesticidas, entre outros, contribuindo para o desenvolvimento de novas cadeias de valor industrial a partir de efluentes orgânicos. Investigadores: Lígia Martins, Sónia Mendes, Vânia Brissos
New enzyme by directed evolution | Researchers turn metallo-oxidase into a laccase
Oeiras, 27.07.2015
Mimicking evolution and natural selection, researchers from the Lab of Microbial and Enzyme Technology turned a metallo-oxidade from the hypertermophile Aquifex aeolicus into a laccase, which is 100-fold more efficient oxidizing organic substrates than metal ions, thus enlarging its range of biotechnological applications. The work is published ahead of print in ACS Catalysis from the American Chemical Society.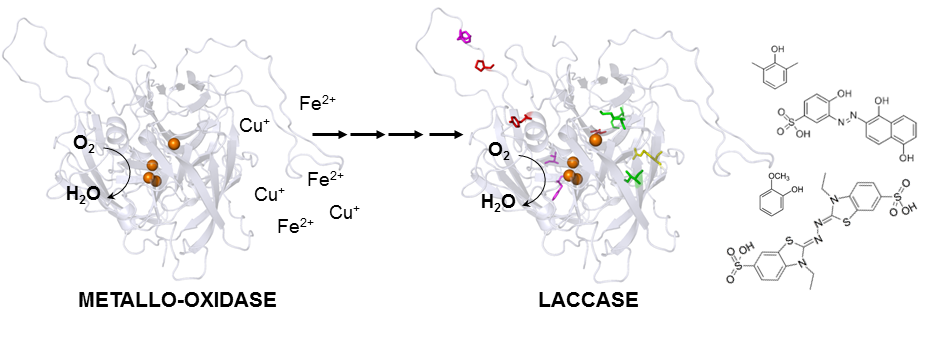
The starting point of this research was an enzyme, functional and stable at very high temperatures (midpoint at 75oC), with the ability to oxidize metals such as Cu(I) and Fe(II). The endpoint is another enzyme, soluble, even more robust, which has the extra ability to oxidize aromatic organic compounds, non-phenolic, phenolic, or synthetic dyes at a higher efficiency than metals. The process that turns the starting point into the endpoint is laboratory-directed evolution. With this technique, the DNA coding for the Aquifex aeolicus metallo-oxidase was randomly mutated, and the mutated enzymes were screened for their ability to oxidize organic compounds. The best-performing enzyme was submitted to an extra round of mutation and selection. After four rounds and 94,000 enzymes tested, researchers ended up with a new laccase: more efficient for degrading aromatic compounds, partially able to oxidize metals, more soluble, and more thermodynamically stable than the original enzyme.
Laccases are considered the “greenest” enzymes in biotechnology because they use oxygen and have water as the only by-product. They find applications in biomass valorization, an alternative to fossil materials for producing chemicals, materials, and fuel. Increasing the substrate range of an intrinsically robust enzyme from a hyperthermophile means increasing the application range in industrial settings. “What these works show is that laboratory-directed evolution is a powerful tool for the modification of biocatalysts and thus for efficient and more sustainable industrial options, which create the XXI century bio-economy, “ says Lígia Martins, who coordinated the study.
Original Paper:
ACS Catalysis (2015) 5: 4932-4941
Turning a Hyperthermostable Metallo-oxidase into a Laccase by Directed Evolution
Vania Brissos , Maura Ferreira , Gregor Grass , and Ligia O. Martins
Laccase is an efficient alternative for the synthesis of heterocyclic compounds | Enzymes for a greener chemistry
Oeiras, 09.09.2014
The cover of the September issue of Green Chemistry, reports on a study involving the Lab of Microbial and Enzyme Technology at ITQB, the Instituto Superior de Engenharia de Lisboa, and the Instituto Superior Técnico. The paper shows how the enzyme laccase, widely studied at ITQB, is an efficient and greener alternative for the chemical oxidatio n of aromatic amines.
n of aromatic amines.
The oxidation products of aromatic amines, heterocyclic phenazines, and phenoxazinones are important biological active motifs of antibiotics and antibacterial agents, anti-tumor agents, pesticides, dyestuffs, and biosensors. They are also the building blocks for the synthesis of organic semiconductors or electrical-photochemical materials. With such a wide range of applications, the bio-oxidation process is of great interest from the biological, chemical, and technological points of view, and efficient, environmentally friendly alternatives are in demand.
Different chemical methods exist for synthesizing these compounds. Still, most suffer from several disadvantages, such as using organic solvents, the requirement for harsh reaction conditions, and low production yields despite some progress. Enzyme-catalyzed processes, on the other hand, are steadily growing as many enzyme-catalyzed reactions meet the criteria of green chemistry. Laccases, in particular, are an attractive option; laccase-catalyzed reactions occur in aqueous systems under mild reaction pH and temperature conditions. The only waste product formed during their reactions is water.
In this work, researchers demonstrated the efficiency of CotA-laccase in mediating the synthesis of a diversity of phenazine and phenoxazinone molecules, which corresponded well to excellent yields. Moreover, the results proposed a pathway for laccase-catalyzed oxidation of substituted aromatic substrates and, as noted in the conclusion of the paper, “a green chemistry process (…) providing a promising approach for the rational synthesis of different heterocyclic scaffolds”.
Original Article:
Green Chem., 2014, 16, 4127-4136
Towards the rational biosynthesis of substituted phenazines and phenoxazinones by laccases
Ana Catarina Sousa, M. Conceição Oliveira, Lígia O. Martins, and M. Paula Robalo
Cleaning-up dyes in the environment | Enzyme biotechnology as an alternative process
Enzymatic dye degradation
Oeiras, 02.10.09
Long-lasting colors in our clothes mean degrading these dyes is difficult. Identifying enzymes that can convert dyes effectively into non-toxic products introduces an attractive alternative for textile effluent clean-up. Two recent papers by the Laboratory of Microbial and Enzyme Technology at ITQB and co-workers show how a recombinant enzyme can transform commercial azo and anthraquinone dyes into environmentally safer compounds.
CotA-laccase is an oxidoreductase present in the spore coat of the bacterium Bacillus subtilis. Its ability to decolorise various synthetic dyes led the researchers to a thorough analysis of the enzymatic oxidative degradation of azo and anthraquinone simple models, namely Sudan Orange G (SOG) and Acid Blue 62 (AB62).
Enzymatic processes were examined using a multidisciplinary approach that combined enzymology, electrochemistry, mass spectrometry (MS), nuclear magnetic resonance (NMR) and microbiology. The researchers identified the reaction intermediates and the main final products and further proposed mechanistic pathways for the oxidation of both types of dyes by laccases.
Azo dyes account for about 50% of all dyes in the textile, food, pharmaceutical, leather, cosmetics, and paper industries. They are, along with anthraquinonic dyes, the most common synthetic colorants released into the environment. Since most synthetic dyes are xenobiotic compounds, researchers also analyzed the potential toxicity of SOG, AB62, and its degradation products using a yeast-based bioassay. After two hours of treatment with CotA-laccase, the inhibitory effect of both dyes on yeast growth was significantly reduced, highlighting the potential for applying this bioremediation-friendly system.
One million tons of synthetic dyes are produced every year. One-tenth of this amount is released to the environment in wastewater, becoming a significant environmental concern. Traditional physicochemical processes for dye removal are expensive, can generate large volumes of sludge, and usually require the addition of environmentally hazardous chemical additives. Resorting to enzyme biotechnology could provide a better solution. Additionally, getting mechanistic insight into the enzymatic dye biotransformation process opens the door for the biosynthesis of novel dye compounds with low toxicological properties.
Original articles
Journal of Biotechnology 2009, 139(1): 68-77
Enzymatic biotransformation of the azo dye Sudan Orange G with bacterial CotA-laccase
Luciana Pereira, Ana V. Coelho, Cristina A. Viegas, Margarida M. Correia dos Santos, Maria Paula Robalo and Lígia O. Martins
Advanced Synthesis & Catalysis (2009), 351 (11-12): 1857 - 1865
On the Mechanism of Biotransformation of the Anthraquinonic Dye Acid Blue 62 by Laccases
Luciana Pereira, Ana V. Coelho, Cristina A. Viegas, Christelle Ganachaud, Gilles Iacazio, Thierry Tron, M. Paula Robalo, Lígia O. Martins