pH induced protein folding/misfolding
pH induced protein folding/misfolding
The human prion protein pH-induced misfolding
The human prion protein (huPrP) is responsible for several neurodegenerative diseases such as Creutzfeldt-Jakob disease (CJD), fatal familial insomnia or Gertsmann-Straussler-Scheinker syndrome. The normal form of the protein, PrPC, misfolds into PrPSc that can form aggregates, which typically accumulate in the brain. According to the protein-only hypothesis, the sole infectious particle is PrPSc which can induce the conversion of PrPC into the infectious form. PrPC (23-231) contains a flexible N-terminal portion (23-124) followed by a globular region (125-228) composed of three α-helices and a small β-sheet. The structure of the misfolded form is unknown, but spectroscopic studies reveal a significant increase in β-sheet (from 3% to 43%) and a decrease in α-helix (from 42% to 30%) [1].
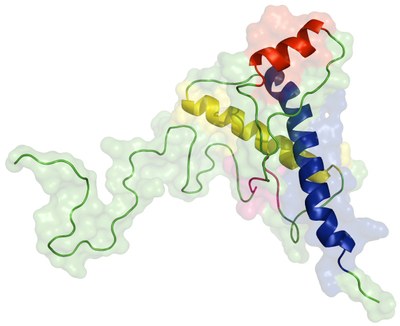
It remains unknown what triggers the misfolding of PrP. One hypothesis is that the low pH of endosomes induces the conversion of PrPC into PrPSc. Experimental and simulation studies show that in fact low pH induces the unfolding of PrPC [2]. The accumulation of PrPSc in endosomes was also observed [3].
We used constant-pH molecular dynamics to study the effect of pH on huPrP structural properties (see here). We observed a strong conformational pH-dependence with clear trends in several properties. In particular, the average helix content decreased towards acidic pH while the average β content increased. In one of the simulations at pH 2, the formation of new β-sheet was particularly striking, with a stable β-rich structure being formed, which can be an intermediate of PrPSc formation.
These interesting observations led us to continue research on this subject. Therefore we have been studying the reversibility of the misfolding shown in the film above when changing the pH to 7 (see here). The most significant effect caused by the change to pH 7 is a global stabilization of the protein structure. Moreover, we observed that some conformational transitions induced by pH 2 were possible to be reverted in many of our simulations, but only in those started from the early moments of the misfolding transition. We also intend to study the aggregation potential of this misfolded structure which can lead to a new model of PrP aggregation.
1. Pan, K.-M., et al. (1993) Proc. Natl. Acad. Sci. USA, 90, 10962.
2. DeMarco, M. L. and Daggett, V. (2005) C. R. Biologies, 328, 847.
3. Arnold, J. E., et al. (1995) J. Pathol., 176, 403.
The human surfactant protein C pH-induced misfolding
Surfactant protein C (SP-C) is a highly hydrophobic protein with two covalently linked fatty acyl chains that adopts a mainly α-helix structure while associated with the membrane. However it misfolds into a β-rich structure under certain environmental conditions, which suggests that SP-C is in a metastable state. The slow α→β transitions are likely to be caused by membrane-dissociation, deacylation and exposure to the neutral pH of the subphase, leading to the formation of amyloid aggregates associated with Pulmonary Alveolar Proteinosis (PAP).
Therefore, we aim to gain insight into pH-dependent conformational behavior of deacylated SP-C in organic solvent, investigating several structural properties of SP-C that might be relevant for the formation of amyloid fibers in PAP.
Publications related with this theme
1. Campos, S. R. R., Machuqueiro, M., Baptista, A. M. (2010) Constant-pH molecular dynamics simulations reveal a β-rich form of the human prion protein. J. Phys. Chem. B, 114:12692-12700.[doi]
2. Vila-Viçosa, D., Campos, S. R. R., Baptista, A. M., Machuqueiro, M. Elucidating the Reversibility of Prion Misfolding: Insights from Constant-pH Molecular Dynamics Simulations, in preparation.



