Single Molecule Processes
Overview
Nanopores are emerging as very efficient single-molecule sensors [1, 2]. At the most simple level, a nanopore is a nanometer-sized hole or pore in an insulating material dividing two reservoirs of buffer. A potential difference is applied across the pore, resulting in a flow of ionic current. The interaction of an analyte with a binding site within the pore, results in a significant change in the pore conductivity. Each single analyte molecule that interacts with the pore will cause a temporary block or reduction in the ionic current. At a fixed potential, the blockade level (extent of the current block) and dwell time (duration of the current block) from each binding event can be used to reveal the identity of the analyte, while the frequency of the binding events reveals the analyte concentration. The physical basis for nanopore-based detection and for the quantification and characterisation of analytes was described by the pioneering work of Bezrukov and Kasianowicz. This resulted in nanopore force spectroscopy using α -haemolysin (α HL) as a biological nanopore [3-7]. Toxic metal ions, drugs, enantiomers, antibiotics and TNT have been among the molecules specifically detected [8-12]. Covalent attachment of different compounds within the pore has been reported for the study of cis-trans isomerization of azobenzene as well as for the multistep formation or breaking of covalent bonds [13, 14]. Polyethylene glycol (PEG) and whole PAMAM dendrimers have also been reported to enter and covalently attach inside α HL [15, 16]. Metal nanoparticles, supramolecular assemblies of a self-assembled monolayer (SAM) into an inorganic nanocrystal, have also been shown to interact with α HL [17, 18].
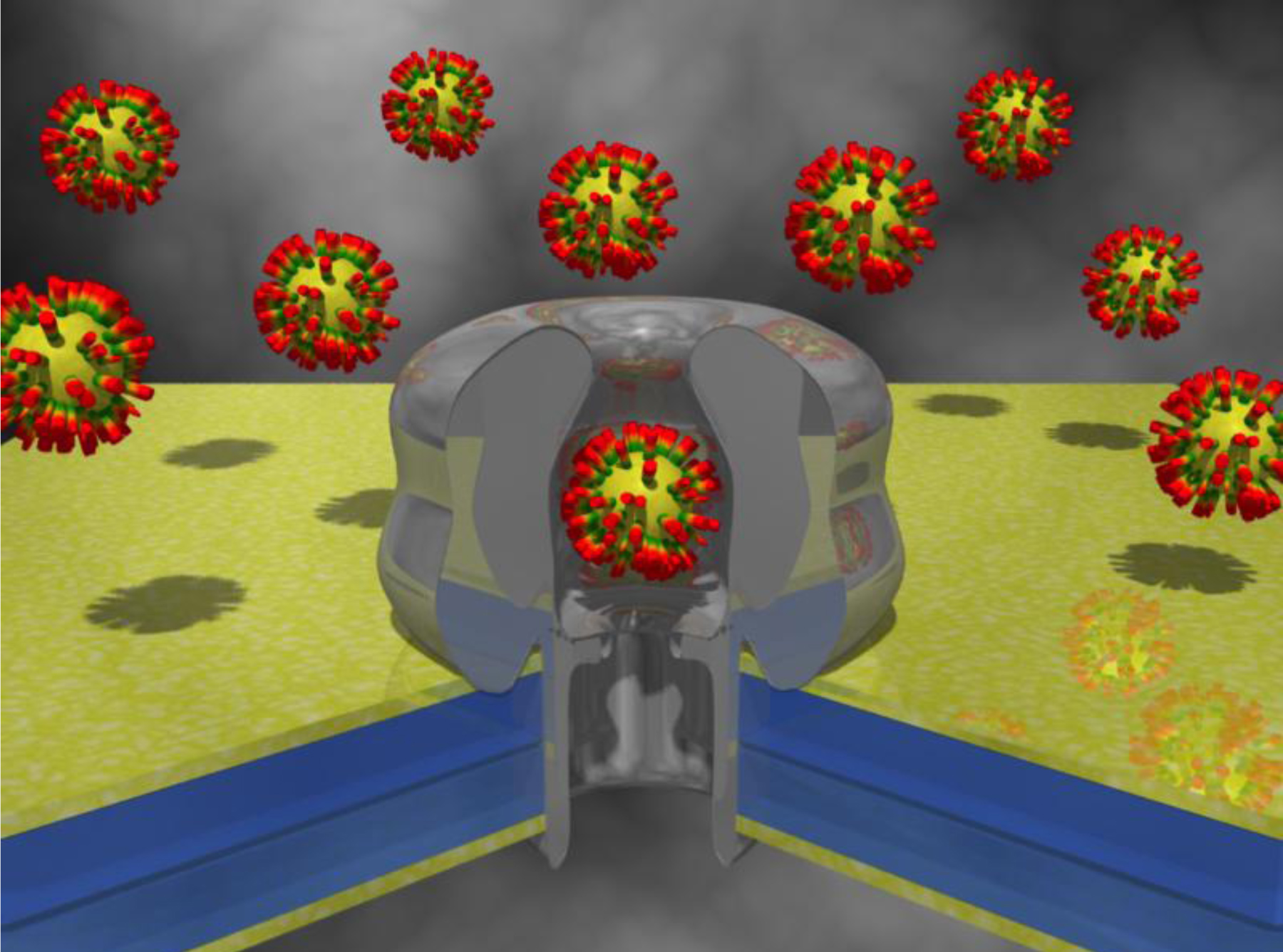
The α HL protein nanopore is an atomically precise tool used routinely in laboratories. Unfortunately, the lipid bilayer into which it is inserted is very sensitive to experimental conditions, in particular to the applied potential difference and is thus unstable. We strive to transfer methods and understanding, obtained from controlled HL experiments, to artificial nanopores. Solid-state nanopore size can be tuned with nanometer precision [19]. These pores exhibit an increased mechanical, chemical, and electrical stability, and thus can be exposed to a wide range of potential, temperature, and solvents. Furthermore, the pore is precisely located, allowing for nanopore containing devices to be precisely assembled.
Free-standing membranes of Si, SiN, SiO2 or graphene are ideal templates for artificial nanopore fabrication [20, 21]. Focussed Ion Beam (FIB) processing technology allows fabrication of nanometer-sized features provided that the membrane can be made homogeneous, insulating, resistant and thin enough [22]. In addition FIB processing of ultra-thin membranes with low mass ions allows for some interesting technical features (autofocussing, generation of sharp edges, little to no redeposition of spluttered material). Silicon nitride (Si3N4) based artificial pores can be reproducibly manufactured down to 2 nm in diameter by focused electron beam perforation of films as thin as 10 nm [20]. These pores can be treated, either chemically or with plasma, in order to display hydrophilic properties, and become permeable to aqueous solutions [21]. Silicon oxide (SiO2) based solid-state nanopores are becoming increasingly popular. They can be manufactured with nanometer precision by drilling with a transmission electron microscope (TEM) or a focused ion beam (FIB) [19]. Silicon oxide nanopores have a hydrophilic surface that favors their interaction with aqueous solutions and also their interaction with biological molecules. Furthermore, they can be covalently modified using a variety of different chemistries (reviewed in [23]). Solid-state nanopores can be covalently modified with antibodies [24] or other molecules to specifically recognise targets of choice.
As a group, our interests lie in the study and development of both biological and solid-state nanopores for applications in defence, civil security, airport security, food safety, monitoring the quality of drinking water, drug detection, drug testing (both performance enhancing and recreational) and medical diagnostics.
1. Healy, K., Nanopore-based single-molecule DNA analysis. Nanomedicine (Lond), 2007. 2(4): p. 459-81.
2. Dekker, C., Solid-state nanopores. Nat Nanotechnol, 2007. 2(4): p. 209-15.
3. Song, L., et al., Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science, 1996. 274(5294): p. 1859-66.
4. Braha, O., et al., Designed protein pores as components for biosensors. Chem Biol, 1997. 4(7): p. 497-505.
5. Bayley, H. and P.S. Cremer, Stochastic sensors inspired by biology. Nature, 2001. 413(6852): p. 226-30.
6. Hornblower, B., et al., Single-molecule analysis of DNA-protein complexes using nanopores. Nat Methods, 2007. 4(4): p. 315-7.
7. Vercoutere, W., et al., Rapid discrimination among individual DNA hairpin molecules at single-nucleotide resolution using an ion channel. Nat Biotechnol, 2001. 19(3): p. 248-52.
8. Gu, L.Q., et al., Stochastic sensing of organic analytes by a pore-forming protein containing a molecular adapter. Nature, 1999. 398(6729): p. 686-90.
9. Kang, X.F., et al., Stochastic detection of enantiomers. J Am Chem Soc, 2006. 128(33): p. 10684-5.
10. Guan, X., et al., Stochastic sensing of TNT with a genetically engineered pore. Chembiochem, 2005. 6(10): p. 1875-81.
11. Astier, Y., O. Braha, and H. Bayley, Toward single molecule DNA sequencing: direct identification of ribonucleoside and deoxyribonucleoside 5'-monophosphates by using an engineered protein nanopore equipped with a molecular adapter. J Am Chem Soc, 2006. 128(5): p. 1705-10.
12. Asandei, A., L. Mereuta, and T. Luchian, The kinetics of ampicillin complexation by gamma-cyclodextrins. A single molecule approach. J Phys Chem B, 2011. 115(33): p. 10173-81.
13. Loudwig, S. and H. Bayley, Photoisomerization of an individual azobenzene molecule in water: an on-off switch triggered by light at a fixed wavelength. J Am Chem Soc, 2006. 128(38): p. 12404-5.
14. Shin, S.H., et al., Kinetics of a reversible covalent-bond-forming reaction observed at the single-molecule level. Angew Chem Int Ed Engl, 2002. 41(19): p. 3707-9; 3523.
15. Movileanu, L., et al., Detecting protein analytes that modulate transmembrane movement of a polymer chain within a single protein pore. Nat Biotechnol, 2000. 18(10): p. 1091-5.
16. Martin, H., et al., Nanoscale protein pores modified with PAMAM dendrimers. J Am Chem Soc, 2007. 129(31): p. 9640-9.
17. Daniel, M.C. and D. Astruc, Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev, 2004. 104(1): p. 293-346.
18. Campos, E., et al., The role of Lys147 in the interaction between MPSA-gold nanoparticles and the alpha-hemolysin nanopore. Langmuir, 2012. 28(44): p. 15643-50.
19. Storm, A.J., et al., Fabrication of solid-state nanopores with single-nanometre precision. Nat Mater, 2003. 2(8): p. 537-40.
20. Li, J., et al., Ion-beam sculpting at nanometre length scales. Nature, 2001. 412(6843): p. 166-9.
21. Wanunu, M. and A. Meller, Chemically modified solid-state nanopores. Nano Lett, 2007. 7(6): p. 1580-5.
22. Gierak, J., et al., Exploration of the ultimate patterning potential achievable with focused ion beams. Ultramicroscopy, 2009. 109(5): p. 457-62.
23. Beaucage, S.L., Strategies in the preparation of DNA oligonucleotide arrays for diagnostic applications. Curr Med Chem, 2001. 8(10): p. 1213-44.
24. Wei, R., et al., Stochastic sensing of proteins with receptor-modified solid-state nanopores. Nat Nanotechnol, 2012. 7(4): p. 257-63.
Dr. James Yates



