UDP-glucose dehydrogenase BceC
The 3D structure determination of BceC led to te elucidation of the mechanistic role of a conserved Tyr in the UDP-glcose dehydrogenase family. This is a project in collaboration with Prof. Arsénio Fialho, IST-IBB, Lisbon, Portugal.
Members of the Burkholderia cepacia complex (BCC) are serious respiratory pathogens in immunocompromised individuals and in patients with cystic fibrosis (CF). They are exceptionally resistant to many antimicrobial agents and have the capacity to spread between patients, leading to a decline in lung function and necrotizing pneumonia. BCC members often express a mucoid phenotype associated with the secretion of the exopolysaccharide (EPS) cepacian. There is much evidence supporting the fact that cepacian is a major virulence factor of BCC.
UDP-glucose dehydrogenase (UGD) is responsible for the NAD-dependent 2-fold oxidation of UDP-glucose (UDP-Glc) to UDP-glucuronic acid (UDP-GlcA), which is a key step in cepacian biosynthesis. Mutagenic studies have been performed on UGDs' active sites and together with available crystallographic structures the catalytic mechanism of this family of sugar nucleotide-modifying enzymes is being elucidated. This family contains a strictly conserved tyrosine residue (Tyr10 in BceC) within the glycine-rich motif (GXGYXG) of its N-terminal Rossmann-like domain. We constructed several BceC Tyr10 mutants, revealing only residual dehydrogenase activity and thus highlighting the importance of this conserved residue in the catalytic activity of BceC and we have determined crystal structures of wild type and Tyr10 BceC mutants. Based on the literature of the UGD/GMD nucleotide sugar 6-dehydrogenase family and the kinetic and structural data we obtained for BceC, we determined Tyr10 as a key catalytic residue in a UGD rate-determining step, the final hydrolysis of the enzymatic thioester intermediate.
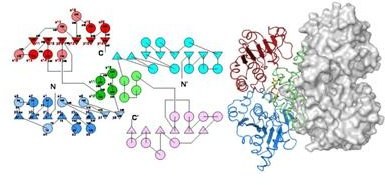 BceC is an homo-dimer (left, its topology diagram, and right, as cartoon plus solvent accessible surface), with each monomer formed by two Rossmann domains (in blue and red, triangles and circles representing beta-strands and alpha-helices, respectively) interleaved by an alpha-helical sub-domain (in green), which is the main responsible for BceC dimerization and where the catalytic apparatus resides. NAD+ is bound twice to the N-terminal domain, and the corresponding NADH products released, accounting for the four electrons oxidation of the alcohol group of glucose C6 centre into the corresponding carboxylic group.
BceC is an homo-dimer (left, its topology diagram, and right, as cartoon plus solvent accessible surface), with each monomer formed by two Rossmann domains (in blue and red, triangles and circles representing beta-strands and alpha-helices, respectively) interleaved by an alpha-helical sub-domain (in green), which is the main responsible for BceC dimerization and where the catalytic apparatus resides. NAD+ is bound twice to the N-terminal domain, and the corresponding NADH products released, accounting for the four electrons oxidation of the alcohol group of glucose C6 centre into the corresponding carboxylic group.
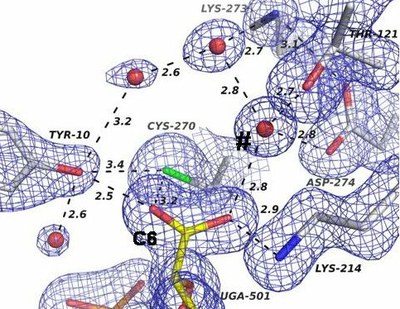 BceC crystallized in presence of the reaction product UDP-GlcA, UGA(501). The carboxylic group at C6 showed hydrogen-bonding interactions with key catalytic residues Lys(214) and Cys(270), to the catalytic water site (#) and to conserved Tyr(10) hydroxyl group. This is at hydrogen bonding distances to SG from Cys(270), and to two water solvent molecules, thus fixing them in the structure. The last step of the hydrogenase catalytic reaction, the hydrolysis of the thioester intermediate by bondage between SG of Cys(270) and C6 of the substrate, may thus be catalyzed by Tyr(10) hydroxyl group, which provides Cys(270) SG directly with a proton thus stabilizing the forming thiolate, the reaction transition state intermediate. The fixation of the two water molecules by Tyr(10) enhances the entropic requirements of the reaction, as corroborated by application of the Eyring-Polanyi equation.
BceC crystallized in presence of the reaction product UDP-GlcA, UGA(501). The carboxylic group at C6 showed hydrogen-bonding interactions with key catalytic residues Lys(214) and Cys(270), to the catalytic water site (#) and to conserved Tyr(10) hydroxyl group. This is at hydrogen bonding distances to SG from Cys(270), and to two water solvent molecules, thus fixing them in the structure. The last step of the hydrogenase catalytic reaction, the hydrolysis of the thioester intermediate by bondage between SG of Cys(270) and C6 of the substrate, may thus be catalyzed by Tyr(10) hydroxyl group, which provides Cys(270) SG directly with a proton thus stabilizing the forming thiolate, the reaction transition state intermediate. The fixation of the two water molecules by Tyr(10) enhances the entropic requirements of the reaction, as corroborated by application of the Eyring-Polanyi equation.



