A bacterial protein for carbon neutrality
(Figure– DvFdhAB structure)
ITQB NOVA researchers Inês Cardoso Pereira and Rita Oliveira and UCIBIO - Chemistry Department FCT NOVA researchers Maria João Romão and Cristiano Mota revealed novel details about a protein capable of reducing carbon dioxide from the atmosphere. The study published in ACS Catalysis provides new leads to solve one of the most urgent challenges for the sustainability of the planet: carbon neutrality.
The CO2 in the Earth’s atmosphere is at the highest level in human history, and several countries around the globe are committed to achieving carbon neutrality by 2050. To achieve that goal, global greenhouse gas emissions must be balanced with carbon dioxide capture. The main natural carbon sinks are soil, forests, and oceans, but these are not enough to reach carbon neutrality. It is crucial to find new catalysts, which can enable the reduction of CO2 into valuable compounds.
In this study. researchers have focused on the biochemical strategies Nature has developed to convert CO2, in order to design more efficient and sustainable catalysts. The metabolism of some microorganisms involves the reduction of CO2. In several anaerobic bacteria, this process is performed by formate dehydrogenases and transforms CO2 into formate, an H2 storage compound and a chemical fuel itself. These enzymes are found in one of the oldest and most energy-efficient biological pathways. “Understanding the functioning of this biological process with billions of years can give us the key to develop new technologies that can reduce atmospheric levels of carbon dioxide", explains ITQB NOVA principal investigator, Inês Cardoso Pereira.

To understand the mechanism of CO2 reduction, researchers investigated the formate dehydrogenase present in Desulfovibrio vulgaris, a bacterium commonly found in marine environments, soils and present in the human intestine. Due to the simple composition and unusual stability of the enzyme, it was possible to unravel novel functional and structural details. This enzyme stands out as a unique model system, combining high CO2 reduction activity with remarkable oxygen stability, both very useful characteristics for biocatalytic applications.
Furthermore, the determination of its three-dimensional structure in different stages of catalysis was decisive towards understanding the mechanism of CO2 reduction. "Its characteristics make this enzyme an ideal model for the study of this mechanism and to inspire the development of simpler synthetic catalysts", says Maria João Romão, from FCT NOVA. The molecular understanding of this mechanism and the characterization of these enzymes provide important clues to advance the development of biotechnological strategies and systems for carbon dioxide fixation.
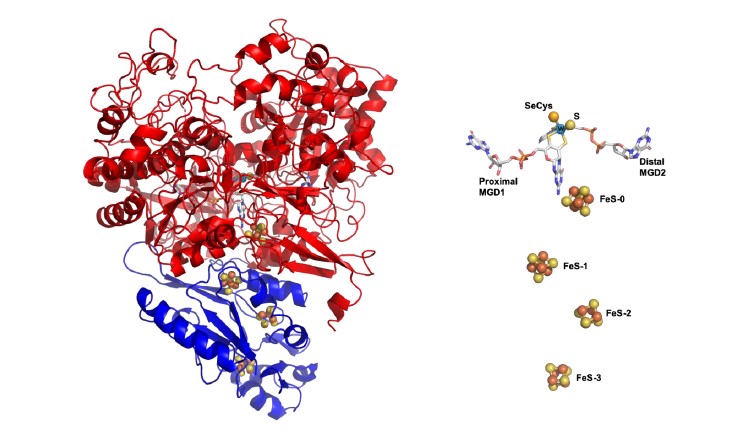
Figure– DvFdhAB structure
The ITQB NOVA team, together with international collaborators, are also involved in other lines of research to apply this enzyme in the development of electrochemical and bio-photovoltaic systems, which directly use electricity or sunlight for CO2 reduction. One such system includes the use of the formate dehydrogenase linked to an electrode that allows the use of CO2 in gaseous form, overcoming mass transport limitations and increasing the catalytic activity and efficiency. In another example they developed systems that combine two enzymes (the formate dehydrogenase and a hydrogenase), to allow direct reduction of CO2 by hydrogen. These developments reinforce the viability of using biohybrid systems in a sustainable decarbonization strategy.
In the news:
- Inês Cardoso Pereira, bioquímica. Ela estudou a proteína capaz de reduzir o CO2 na atmosfera, in TSF, 24.03.2020
- Portugueses identificam funcionamento de bactéria que absorve CO2 e produz combustível, in Expresso, 06.03.2020
- Investigadores portugueses identificam proteína que reduz CO2 na atmosfera, in Observador, 07.03.2020
- Investigadores da Nova identificam proteína que reduz CO2 na atmosfera, in RTP, 07.03.2020
- Investigadores da Nova identificam proteína que reduz CO2 na atmosfera, Notícias ao Minuto
- Há uma nova esperança para reduzir o CO2 na atmosfera?, in TVI, 07.03.2020
-
Investigadores da Nova identificam proteína que reduz CO2 na atmosfera, in Sábado, 07.03.2020
-
Investigadores da Universidade Nova de Lisboa identificam proteína que reduz dióxido de carbono na atmosfera, in Correio da Manhã, 07.03.2020
-
Investigadores da Nova identificam proteína que reduz CO2 na atmosfera, in Porto Canal , 07.03.2020
-
Investigadores da Nova identificam proteína que reduz CO2 na atmosfera, in Açoriano Oriental , 07.03.2020
-
Investigadores da Nova identificam proteína que reduz CO2 na atmosfera, in Postal Algarve , 07.03.2020
-
Investigadores da Nova descobrem processo inovador a nível mundial para atingir a neutralidade carbónica, in O instalador , 10.03.2020
Original paper
Toward the mechanistic understanding of enzymatic CO2 reduction
Ana Rita Oliveiraǂ, Cristiano Motaǂ, Cláudia Mourato, Renato M. Domingos, Marino F. A. Santos, Diana Gesto, Bruno Guigliarelli, Teresa Santos-Silva, Maria João Romão*, Inês A. Cardoso Pereira*
ACS Catalysis, DOI 10.1021/acscatal.0c00086
Related publications from the ITQB NOVA team and collaborators:
Electroenzymatic CO2 Fixation Using Redox Polymer/Enzyme-Modified Gas Diffusion Electrodes, Szczesny J, Ruff A, Oliveira AR, Pita M, Pereira IAC, De Lacey AL, Schuhmann W
ACS Energy Lett., 2020 5, 321-327 DOI 10.1021/acsenergylett.9b02436
Reversible and Selective Interconversion of Hydrogen and Carbon Dioxide into Formate by a Semiartificial Formate Hydrogenlyase Mimic
Sokol KP, Robinson WE, Oliveira AR, Zacarias S, Lee C-Y, Madden C, Bassegoda A, Hirst J, Pereira IAC, Reisner E
J. Am. Chem. Soc., 2019, 141 (44), 17498-17502 DOI 10.1021/jacs.9b09575
Interfacing Formate Dehydrogenase with Metal Oxides for the Reversible Electrocatalysis and Solar-Driven Reduction of Carbon Dioxide
Miller, M, Robinson, WE, Oliveira, AR, Heidary, N, Kornienko, N, Warnan, J, Pereira, IAC, Reisner, E
Angew. Chemie Int. Ed. 2019, 131 (14), 4649-4653 DOI: 10.1002/anie.201814419
Photoreduction of CO2 with a Formate Dehydrogenase Driven by Photosystem II Using a Semi-artificial Z-Scheme Architecture
Sokol KP, Robinson WE, Oliveira AR, Warnan J, Nowaczyk MM, Ruff A, Pereira IAC, Reisner E
J. Am. Chem. Soc., 2018, 140, 16418-16422 DOI: 10.1021/jacs.8b10247







