Journal of Bacteriology features enzyme structure
Oeiras, 10.03.09
The cover of last February issue of Journal of Bacteriology features the tridimensional structure of an enzyme from the human pathogen Klebsiella pneumoniae as determined by X-ray crystallography. The authors of this study integrate the Macromolecular Crystallography Unit – Structural Genomics Laboratory, headed by Maria Arménia Carrondo.
Klebsiella pneumoniae is a nosocomial pathogen frequently isolated from opportunistic infections, especially in clinical environments. But in spite of its potential pathogenicity, this microorganism can have a useful role.
|
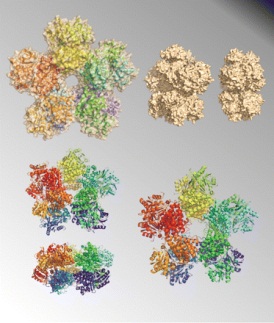
Cover image of J. Bacteriol 191(4) |
The featured enzyme is 1,3-propanediol dehydrogenase, the key enzyme in the microbial production of 1,3-propanediol, which is an important organic compound used both in the chemical synthesis of polymers and as a bulk chemical in the cosmetic, food, and drug industries. The article on page 1143 describes how unlike the structures of other members of this family of enzymes, 1, 3- propanediol dehydrogenase from K. pneumoniae revealed an unprecedented decameric arrangement, which may be relevant for the enzymatic activity of this protein in solution. The cover figure depicts several spatial orientations and model representations (ribbons and molecular surface) of the decamer.
|
1,3-Propanediol Dehydrogenase from Klebsiella pneumoniae: Decameric Quaternary Structure and Possible Subunit Cooperativity
David Marçal, Ana Toste Rêgo, Maria Arménia Carrondo, and Francisco J. Enguita
Journal of Bacteriology 191 (4): 1143-1151, doi:10.1128/JB.01077-08