The subtle connection between a tumor virus and leukemia
Kaposi’s sarcoma-associated herpesvirus (KSHV) is one of seven currently known human cancer viruses, or oncoviruses, and a leading cause of malignancy in AIDS. KSHV establishes persistent, lifelong infection of its human host and persists latently in tumor cells. In a new study, researchers revealed how virus latency is linked to the Mixed-lineage leukemia 1 protein.
During latency, gammaherpesviruses, such as KSHV, persist as multicopy, circularized genomes in the cell nucleus (episome) and express a small subset of viral genes. KSHV latency-associated nuclear antigen (LANA) is the predominant gene expressed. LANA, plays a critical role during latent infection and causes several KSHV associated malignancies. Upon infection, the viral genome is rapidly transported to the nucleus, where it associates with histone proteins to form chromatin, this compacts the viral DNA and plays a role in replication and life cycle of the virus. This process involves several interactions with host proteins, such as Mixed Lineage Leukemia 1 (MLL1), and leads to chromatin modifications. MLL1 is a histone methyltransferase, and when deregulated is related to pediatric and adult leukemia.
Researchers at ITQB NOVA, together with scientists from the Harvard Medical School in collaboration with scientists at the Instituto de Medicina Molecular, the Shanghai Institute of Biochemistry and Cell Biology, University of Chinese Academy of Sciences, demonstrated in a paper published in Nucleic Acids Research that MLL1 has a unique role in KSHV infection.
By combining in vitro and cell line studies, fluorescence microscopy, and biophysical methods, it was possible to unravel several details of this interaction. The alterations in the levels of an epigenetic marker on the KSHV terminal repeat (TR) DNA induced by MLL1 following infection, combined with its activity at additional KSHV genomic sites, are key for latency establishment. Besides, MLL1 may act at host genes that are critical for the promotion and maintenance of latency. Once epigenetic marks are established, the viral genome persists as a chromatinized episome, and LANA may function with the MLL1 complex to maintain the marks and drive viral latency.
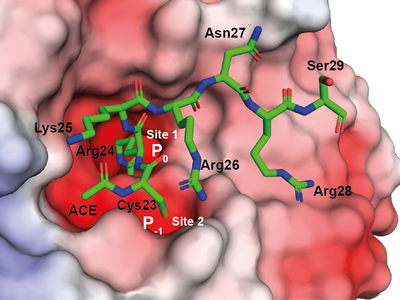 Through the description of the crystal structure of a LANA peptide complexed with WDR5, a component of the MLL1 complex, researchers revealed a potential regulatory mechanism. When the leukemia-related protein expression is disrupted, the capacity of KSHV to develop latency is extremely poor.
Through the description of the crystal structure of a LANA peptide complexed with WDR5, a component of the MLL1 complex, researchers revealed a potential regulatory mechanism. When the leukemia-related protein expression is disrupted, the capacity of KSHV to develop latency is extremely poor.
This discovery represents an important step in the effort for the prevention and treatment of KSHV malignancy. “By understanding in detail how the KSHV LANA protein and MLL1 interact, it is possible to develop strategies to disrupt MLL1 regulation and function”, says Colin McVey, head of the Structural Virology Lab.
The work was supported by the National Institutes of Health (NIH), FCT (Fundação para a Ciência e Tecnologia), and performed within the projects funded by FEDER funds under the PT2020, funding iNOVA4Health Research Unit.
Click on the coloured circles to see the interaction.
Original Paper
Nucleic Acids Research: https://doi.org/10.1093/nar/gkab1094
MLL1 is regulated by KSHV LANA and is important for virus latency
Min Tan, Shijun Li, Franceline Juillard, Rute Chitas, Tânia F. Custódio, Han Xue, Agnieszka Szymula, Qiming Sun, Bing Liu, Ángel L. Álvarez, She Chen, Jing Huang, J. Pedro Simas*, Colin E. McVey* and Kenneth M. Kaye*







