Proteins partner for crucial cellular quality control system
Dysfunctional ribosomes are associated with severe human genetic diseases known as ribosomopathies, such as skeletal muscle atrophy and some anemias. Muscular atrophy can occur in diseases such as diabetes, cancer and heart failure, among others. Many reasons may cause ribosomes to malfunction and threaten the survival of cells.
Ribosomes are made of proteins and RNA, more specifically ribosomal RNA, or rRNA. RNA is a nucleic acid, like DNA, and is crucial to all known life forms. Ribosomes are responsible for protein synthesis in all living organisms.
Researchers from ITQB NOVA Cecília Arraiano´s Control of Gene Expression Lab concluded for the first time that two RNA-binding proteins, Hfq and Ribonuclease R, cooperate in a previously unknown quality control mechanism. This surveillance system is associated with correct ribosome assembly and the elimination of detrimental rRNA fragments. The study was published today in mBio, ASM's first broad-scope scientific journal which publishes the best research in microbiology and allied fields.
As the name suggests, RNA-binding proteins are proteins that bind to RNA and have essential regulatory functions. In 2018, Cecília Arraiano’s team had already identified the key role of Hfq in this surveillance process, critical for the integrity of the functional ribosome. Now, the researchers demonstrated that Hfq and Ribonuclease R (RNase R) can associate and may act independently or as part of a complex. This partnership governs the degradation and processing of ribosomal RNA molecules and was previously unknown.
The results show that the RNA chaperone Hfq and the degradative enzyme RNase R are involved in the correct processing of rRNA precursors – from which rRNA originates. Furthermore, they contribute to the elimination of aberrant ribosomal RNAs. Hfq and RNase R manage previously unidentified processing and degradation pathways, which are crucial for cell survival.
“The surveillance mechanism mediated by Hfq and RNase R is active in the maturation of rRNA precursors and is highly advantageous for the cell, preventing translational errors and superfluous energetic costs”, explains researcher Ricardo dos Santos. This protein partnership in the metabolic pathways of highly structured and stable RNAs “may represent a broader mechanism of RNA quality control, given the high conservation of these RNA-binding proteins throughout evolution”, adds José Andrade, while Cecília Arraiano underlines: “This discovery can have important implications for biotechnology regarding protein synthesis and biomedical studies in ribosomopathies”.
This study was developed within the scope of Ricardo dos Santos’ PhD thesis at ITQB NOVA, with funding from FCT.
ITQB NOVA researchers Ricardo Santos, José Andrade and Cecília Arraiano.
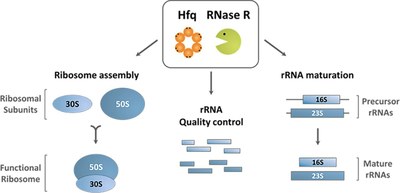
Model for the roles of Hfq and RNase R cooperation in RNA quality control.
Original paper:
American Society for Microbiology | mBio 02398-20, vol. 11, no. 5
Hfq and RNase R mediate rRNA processing and degradation in a novel RNA quality control process
Ricardo F. dos Santos1+, José M. Andrade1,+#, Joana Pissarra1,*, Murray P. Deutscher2 and Cecília M. Arraiano1,#
1. Instituto de Tecnologia Química e Biológica António Xavier, Universidade Nova de Lisboa, Avenida da República, 2780-157 Oeiras, Portugal
2. Department of Biochemistry and Molecular Biology, Miller School of Medicine, University of10 Miami, Miami, FL 33101, USA
* Present address: Institut de Recherche Pour Le Développement (IRD), UMR INTERTRYP IRD12 CIRAD, University of Montpellier, F-34398 Montpellier, France
#Co-corresponding authors
+Joint first authors. Author order was determined in order of increasing seniority.







