DNA repair
If damaged bases in our genome are not repaired they can cause mutations, replication errors and persistent DNA damage which ultimately can lead to cancer and premature aging. Base damages are repaired in the base excision repair (BER) pathway which is highly conserved from bacteria to man. BER is initiated by DNA glycosylases which removes deaminated, alkylated and oxidized bases, abasic sites and single-strand breaks. The BER process consists of three steps 1) base recogntion and removal, 2) DNA processing and 3) base replacement (see figure below). Upon recognition of the damaged bases the glycosylases flip them out of the DNA and into the catalytic pocket and removes them by hydrolysing the N-glycosidic bond between the base and the sugar phosphate backbone, thereby generating an abasic site (AP-site). This site is further processed by AP-endonucleases followed by DNA polymerases and DNA ligases which clean the ends, introduce the correct base and seal the gap. There are two groups of DNA glycosylases, monofunctional which remove the base from the DNA and bifunctional which both remove the base and makes a nick in the DNA after base removal. There are also two paths of repair, the dominant short patch repair (also called single nucleotide repair) where one base is removed and replaced and the long patch repair where one base is removed but where a longer strech of DNA is replaced. The BER pathway is illustrated in the figure below.
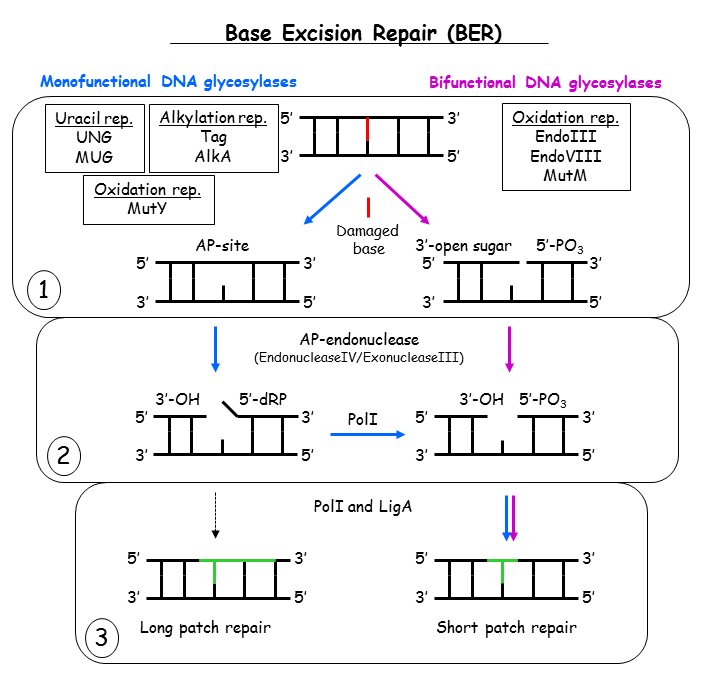
So far we have determined crystal structures of seven uracil DNA glycosylases (six UNGs and one MUG), including complexes with DNA and inhibitor, one alkylation repair glycosylase (AlkA), three oxidation repair enzymes (two Endonuclease III and one MutT) and one replication protein (beta-clamp). They are all characterised by using biochemical, biophysical or spectroscopic methods, and have led to an increased understanding of the molecular mechanisms underlying DNA repair.
Lately we have also studied the role of the iron-sulfur cluster (FeS) of Endonuclease III (EndoIII) enzymes in DNA repair, and by employing advanced spectroscopic and electrochemical methods we have suggested that the current model of FeS cluster involvement in DNA repair needs revision. We are currently following up these results and are developing projects to study the largely unknown BER initiation process by using the EndoIII enzymes and other DNA glycosylases as model enzymes and single molecule techniques.



